Can Acetone Form Hydrogen Bonds
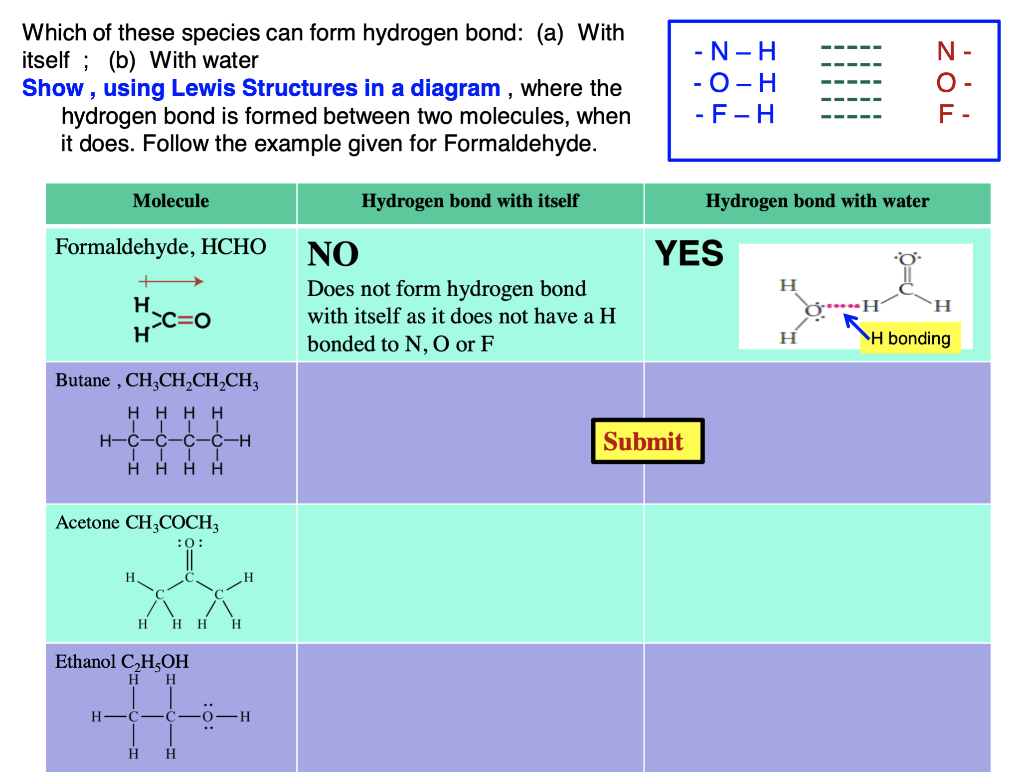
Can Acetone Form Hydrogen Bonds - Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. Four images are given which show an acetone molecule interacting with a water. Acetone can form hydrogen bonds with water. The water molecule forms two hydrogen bonds with acetone: I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,.
The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Four images are given which show an acetone molecule interacting with a water. The water molecule forms two hydrogen bonds with acetone: I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Acetone can form hydrogen bonds with water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming.
Acetone can form hydrogen bonds with water. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. The water molecule forms two hydrogen bonds with acetone: Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. Four images are given which show an acetone molecule interacting with a water.
SOLVED2. Can a molecule of acetone form a hydrogen bond with water
Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The water molecule forms two hydrogen bonds with acetone: The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,..
SOLVED How many hydrogen bonds can form between an acetone molecule
Acetone can form hydrogen bonds with water. The water molecule forms two hydrogen bonds with acetone: The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Acetone does not have a hydrogen atom bonded to a highly electronegative atom,.
hydrogen bond acetone ethanol YouTube
The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Four images are given which show an acetone molecule interacting with a water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the.
Answered Can Butane, Acetone, and Ethanol form a… bartleby
I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. The water molecule forms two hydrogen bonds with acetone: Four images are given which show an acetone molecule interacting with a water. Acetone does not have a hydrogen atom.
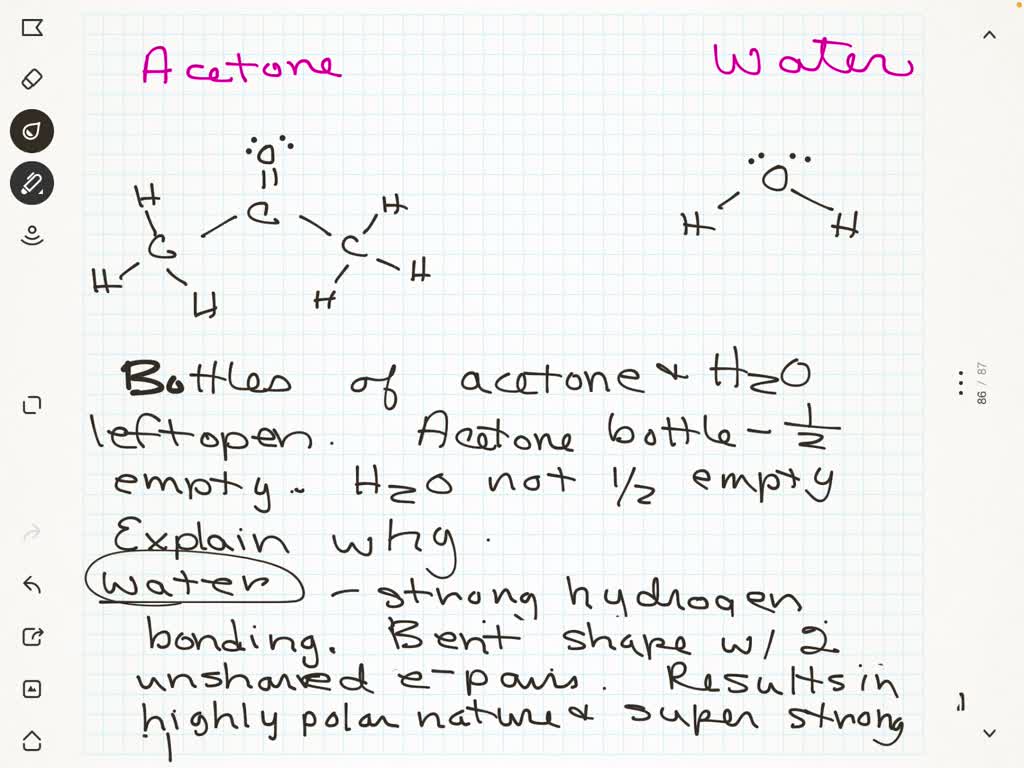
I had to draw Hbonds between water and Acetone. Did I do it correctly
Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. Acetone can form hydrogen bonds with water. The water molecule forms two hydrogen bonds with acetone: I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The hydrogen atom of h 2 o is.
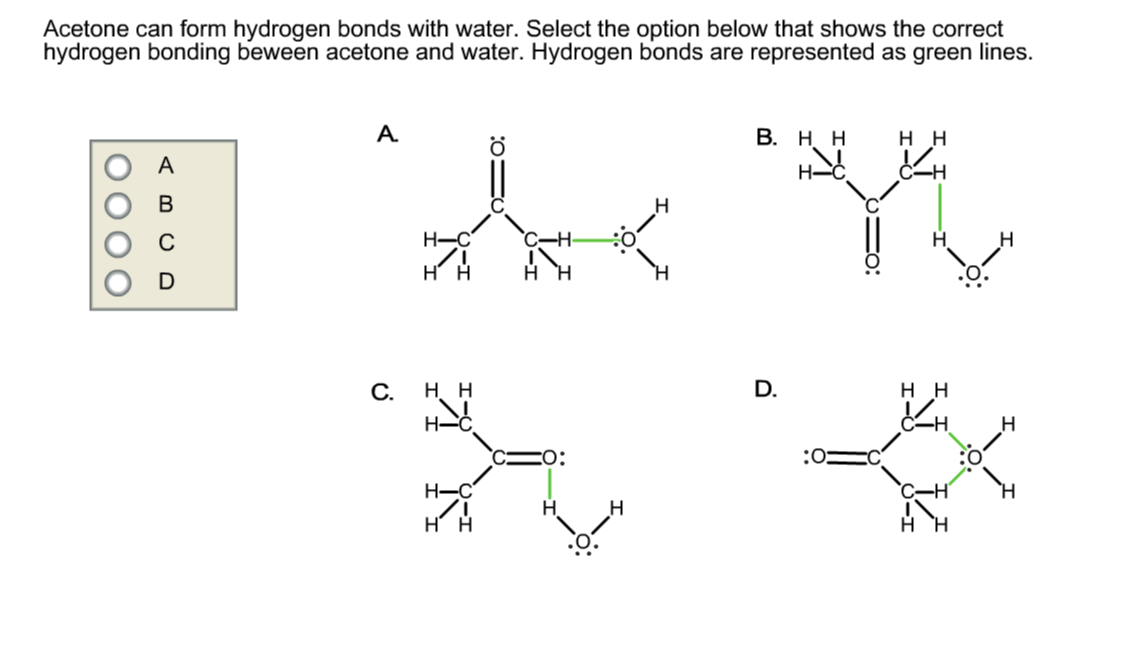
Solved Acetone can form hydrogen bonds with water. Select
The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Acetone can form hydrogen bonds with water. The water molecule forms two hydrogen bonds with acetone: Four images are given which show an acetone molecule interacting with a water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the.
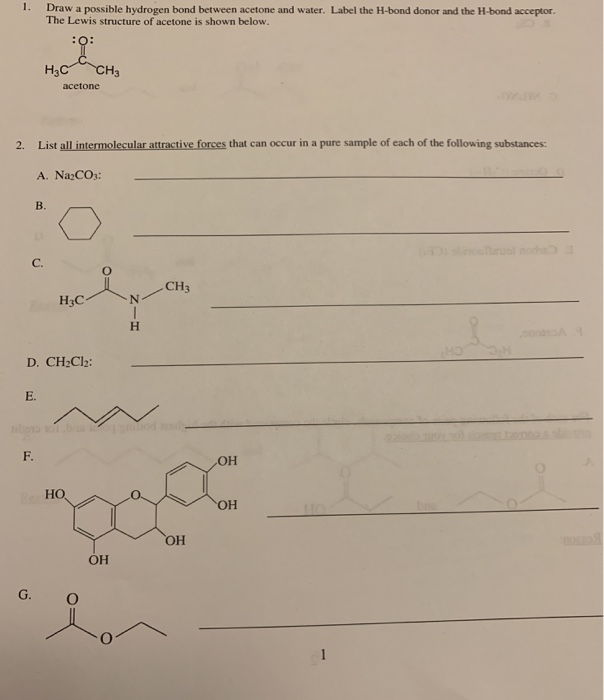
Solved 1. Draw a possible hydrogen bond between acetone and
I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Four images are given which show an acetone molecule interacting with a water. The water molecule forms two hydrogen bonds with acetone: Acetone does not have a hydrogen atom.
SOLVED SOURCE CHAPTER PROBLEM TOPIC INTRAMOLECULAR FORCES The
I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The water molecule forms two hydrogen bonds with acetone: Four images are given which show an acetone molecule interacting with a water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. Acetone can.
Acetone Lewis Structure With Polarity
The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The water molecule forms two hydrogen bonds with acetone: Acetone can form hydrogen bonds with water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom,.
SOLVEDWhy can't two molecules of acetone form a hydrogen bond with
The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Acetone can form hydrogen bonds with water. Four images.
The Hydrogen Atom Of H 2 O Is Coordinated To The Carbonyl Group Of Acetone,.
Four images are given which show an acetone molecule interacting with a water. Acetone can form hydrogen bonds with water. The water molecule forms two hydrogen bonds with acetone: Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming.