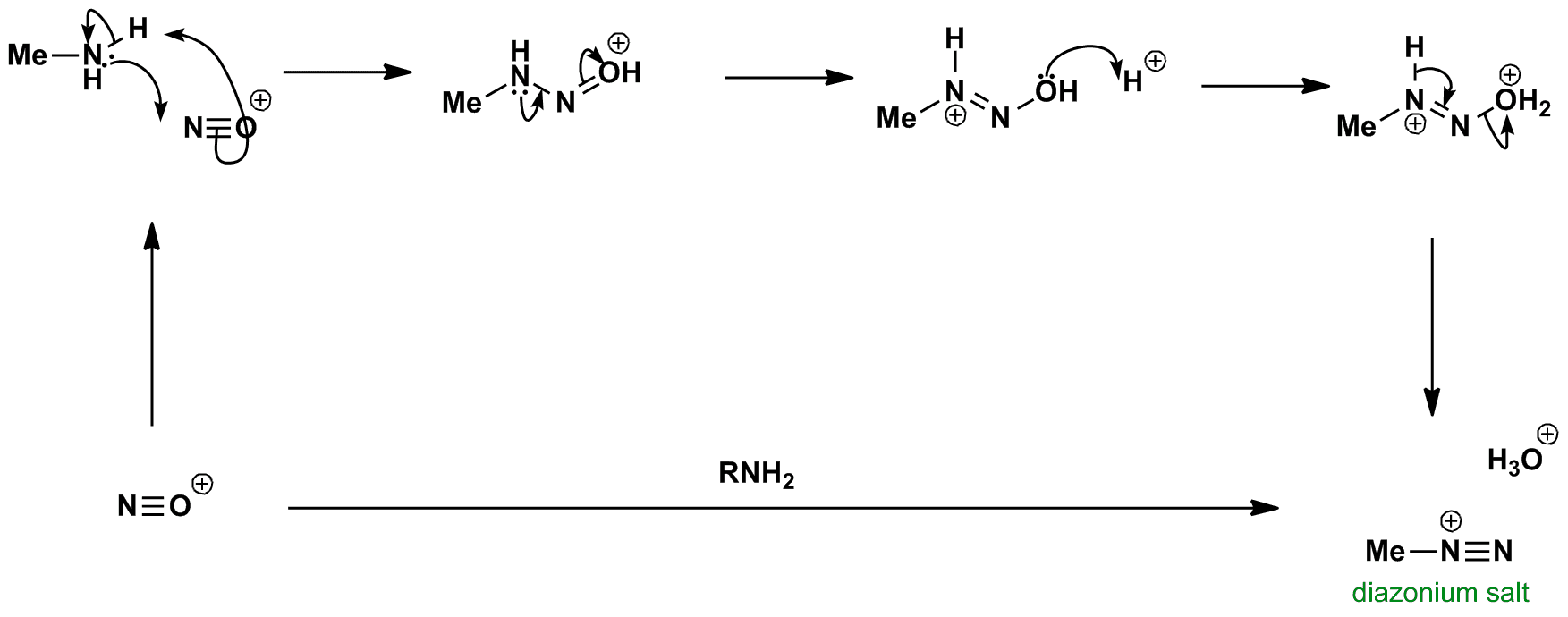
Diazonium Salt Formation
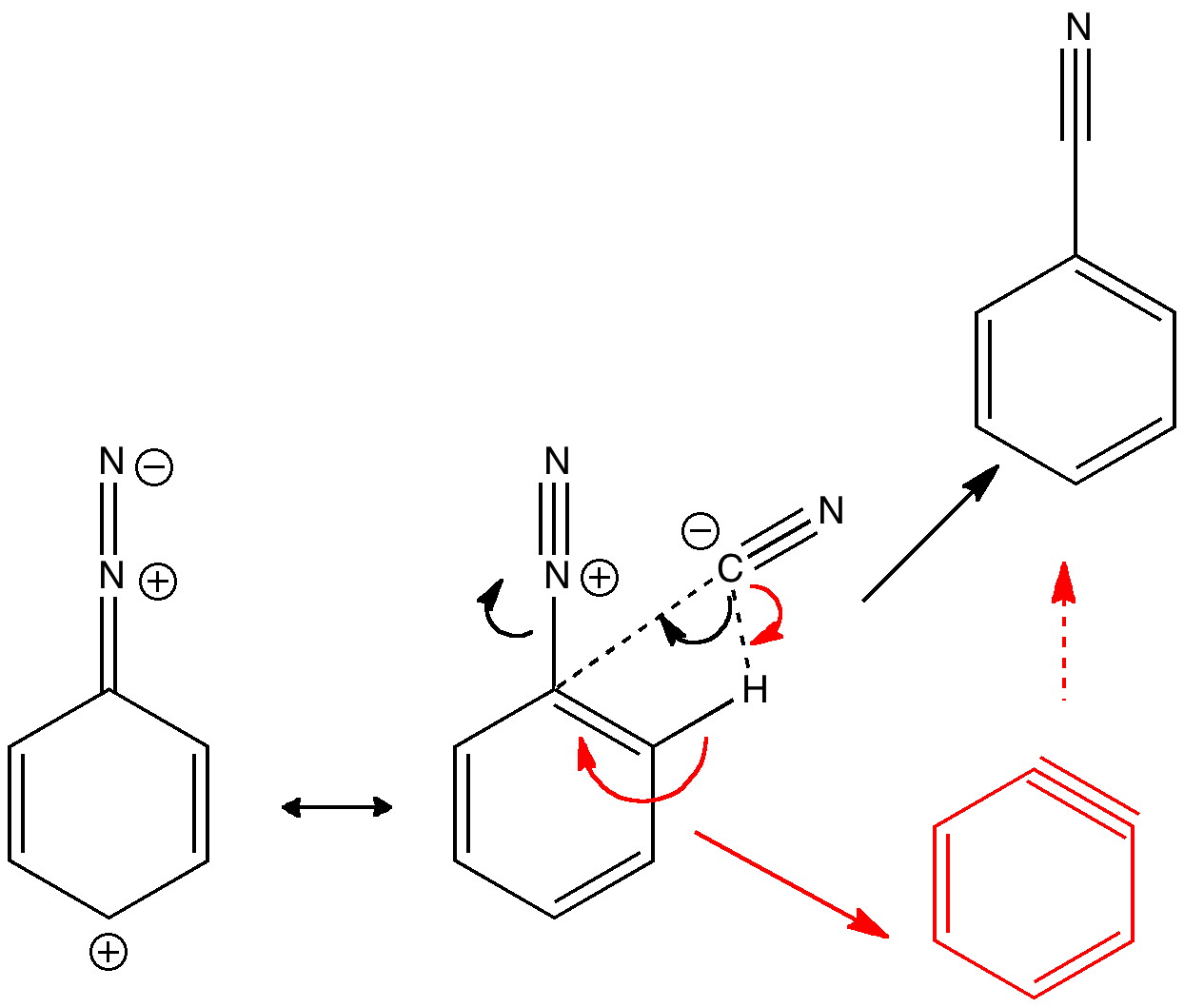
Diazonium Salt Formation - The aryl cation is highly reactive and attacked by the aryl ring that leads to. One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. One of the most common methods of preparation of diazonium salt is by reacting nitrous acid with aromatic amines. The diazonium salt is believed to decompose into aryl cation and nitrogen.
The aryl cation is highly reactive and attacked by the aryl ring that leads to. One of the most common methods of preparation of diazonium salt is by reacting nitrous acid with aromatic amines. One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. The diazonium salt is believed to decompose into aryl cation and nitrogen.
The diazonium salt is believed to decompose into aryl cation and nitrogen. One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. The aryl cation is highly reactive and attacked by the aryl ring that leads to. One of the most common methods of preparation of diazonium salt is by reacting nitrous acid with aromatic amines.
Janus mechanisms (the past and the future) Reactions of the diazonium
One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. One of the most common methods of preparation of diazonium salt is by reacting nitrous acid with aromatic amines. The aryl cation is highly reactive and attacked by the aryl ring that leads to. The diazonium salt is believed.
Discover the Fascinating Diazotization Mechanism
One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. One of the most common methods of preparation of diazonium salt is by reacting nitrous acid with aromatic amines. The diazonium salt is believed to decompose into aryl cation and nitrogen. The aryl cation is highly reactive and attacked.
Substrate scope of diazonium salts Reaction conditions 1al (1.5 mmol
The diazonium salt is believed to decompose into aryl cation and nitrogen. One of the most common methods of preparation of diazonium salt is by reacting nitrous acid with aromatic amines. One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. The aryl cation is highly reactive and attacked.
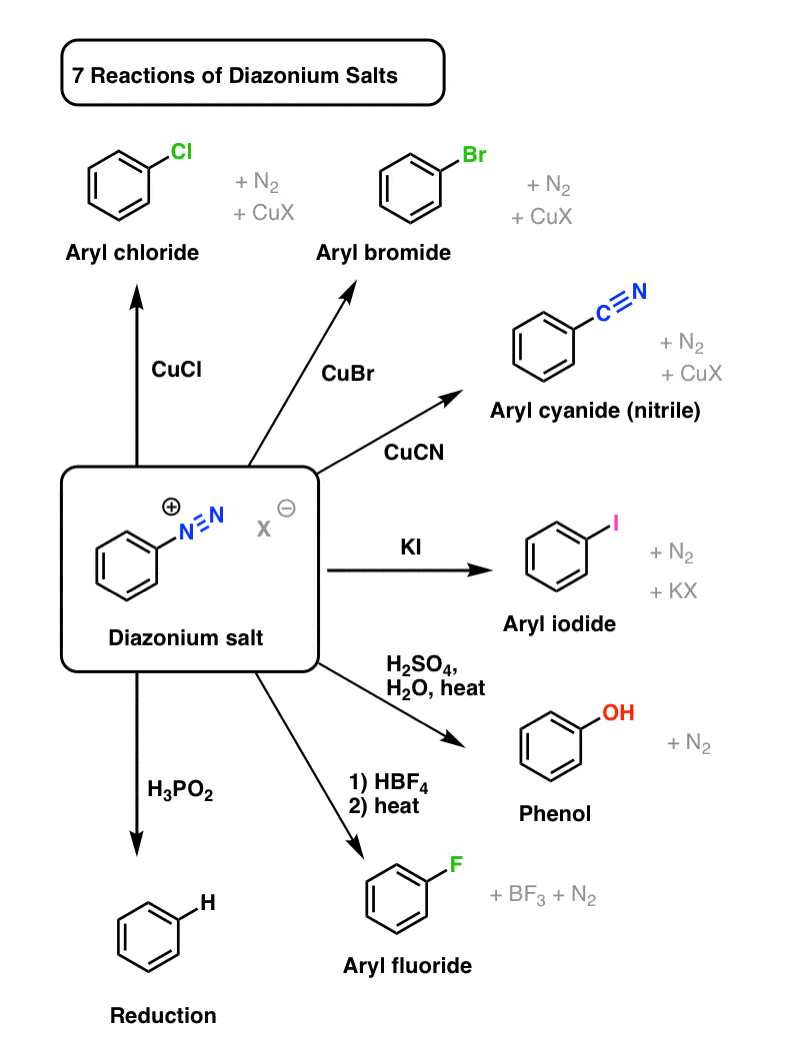
Reactions of Diazonium Salts Sandmeyer and Related Reactions
One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. The diazonium salt is believed to decompose into aryl cation and nitrogen. One of the most common methods of preparation of diazonium salt is by reacting nitrous acid with aromatic amines. The aryl cation is highly reactive and attacked.
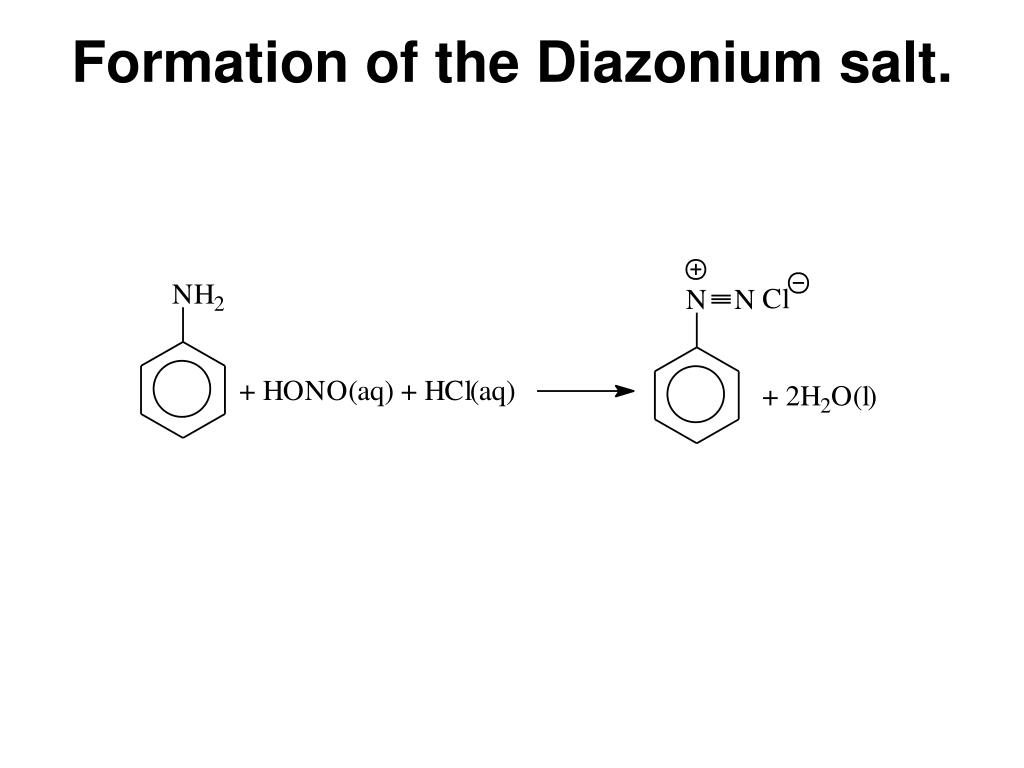
Formation of Diazonium Salts ChemistryScore
The aryl cation is highly reactive and attacked by the aryl ring that leads to. One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. The diazonium salt is believed to decompose into aryl cation and nitrogen. One of the most common methods of preparation of diazonium salt is.
Reactions of Diazonium Salts Sandmeyer and Related Reactions
The aryl cation is highly reactive and attacked by the aryl ring that leads to. One of the most common methods of preparation of diazonium salt is by reacting nitrous acid with aromatic amines. The diazonium salt is believed to decompose into aryl cation and nitrogen. One of the most common methods of preparation of diazonium salt is by the.
DIAZONIUM Chemistry in 2020 Organic chemistry study, Organic
The diazonium salt is believed to decompose into aryl cation and nitrogen. The aryl cation is highly reactive and attacked by the aryl ring that leads to. One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. One of the most common methods of preparation of diazonium salt is.
PPT Diazonium Salts PowerPoint Presentation, free download ID1110651
The diazonium salt is believed to decompose into aryl cation and nitrogen. The aryl cation is highly reactive and attacked by the aryl ring that leads to. One of the most common methods of preparation of diazonium salt is by reacting nitrous acid with aromatic amines. One of the most common methods of preparation of diazonium salt is by the.
Formation of Diazonium Salts ChemistryScore
The aryl cation is highly reactive and attacked by the aryl ring that leads to. One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. The diazonium salt is believed to decompose into aryl cation and nitrogen. One of the most common methods of preparation of diazonium salt is.
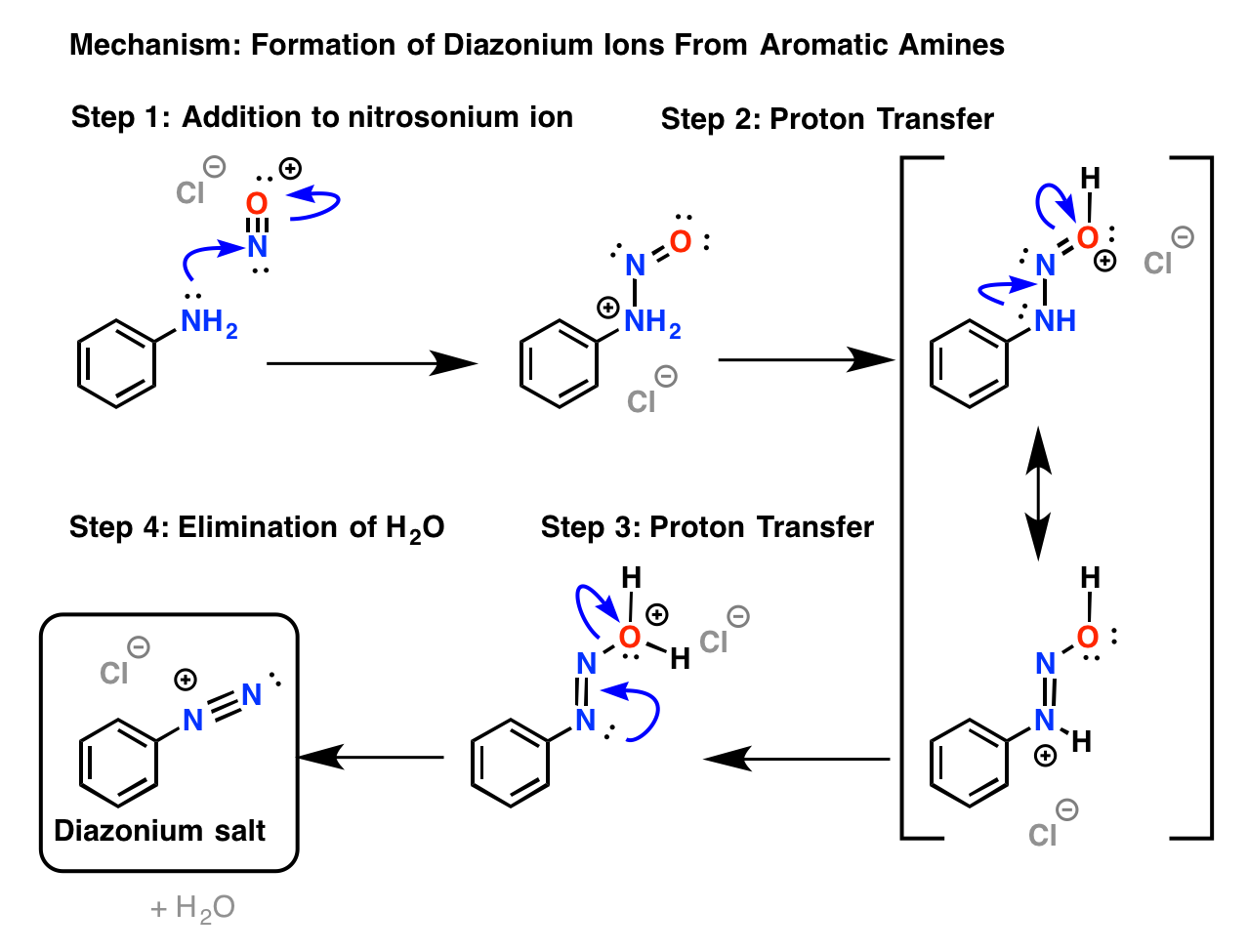
Formation of Diazonium Salt Diazotization
The aryl cation is highly reactive and attacked by the aryl ring that leads to. The diazonium salt is believed to decompose into aryl cation and nitrogen. One of the most common methods of preparation of diazonium salt is by the reaction of nitrous acid with aromatic amines. One of the most common methods of preparation of diazonium salt is.
One Of The Most Common Methods Of Preparation Of Diazonium Salt Is By The Reaction Of Nitrous Acid With Aromatic Amines.
The diazonium salt is believed to decompose into aryl cation and nitrogen. One of the most common methods of preparation of diazonium salt is by reacting nitrous acid with aromatic amines. The aryl cation is highly reactive and attacked by the aryl ring that leads to.