Ipledge Rems Fact Sheet
Ipledge Rems Fact Sheet - To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. A rems is a risk management program that uses risk. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. To avoid serious risks to unborn babies, the food and drug administration (fda) has required a special program called a risk evaluation and. What is the ipledge program? The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. What is a risk evaluation and mitigation strategy (rems)? What is the ipledge rems? The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure.
To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. A rems is a risk management program that uses risk. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. To avoid serious risks to unborn babies, the food and drug administration (fda) has required a special program called a risk evaluation and. What is the ipledge program? What is a risk evaluation and mitigation strategy (rems)? To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge rems?
To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge program? A rems is a risk management program that uses risk. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. To avoid serious risks to unborn babies, the food and drug administration (fda) has required a special program called a risk evaluation and. The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. What is a risk evaluation and mitigation strategy (rems)? What is the ipledge rems?
Accutane Ipledge Registration
To avoid serious risks to unborn babies, the food and drug administration (fda) has required a special program called a risk evaluation and. A rems is a risk management program that uses risk. What is the ipledge rems? To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge.
IPledge Isotretinoin REMS Prompts FDA Flexibility & Plea to Manufacturers
The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. What is a risk evaluation and mitigation strategy (rems)? The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. To avoid serious risks to unborn babies, the food and drug.
Petition · Cancel iPLEDGE REMS Program so that isotretinoin patients
To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is a risk evaluation and mitigation strategy (rems)? The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda).
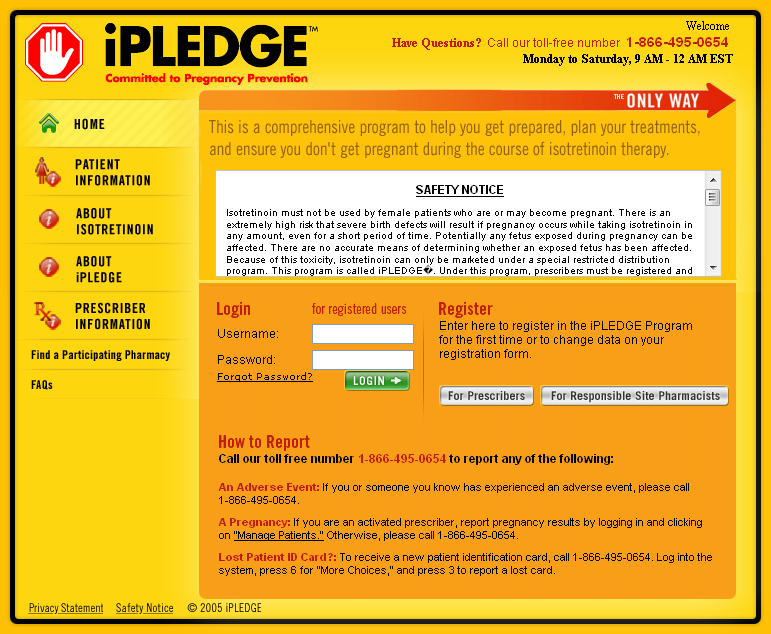
iPledge Login First Time at ️ [2023]
To avoid serious risks to unborn babies, the food and drug administration (fda) has required a special program called a risk evaluation and. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special..
FDA Issues Updates to iPLEDGE® REMS Program PAAS National
To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge rems? A rems is a risk management program that uses risk. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. What is the ipledge program?
Fillable Online iPledge REMS Frequently Asked Questions (FAQ
To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. A rems is a risk management program that uses risk. To avoid serious risks to unborn babies, the food and drug.
iPLEDGE® REMS Accutane®
The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is a risk evaluation and mitigation strategy (rems)? A rems is a risk management program that uses risk. To avoid serious risks.
iPLEDGE allows athome pregnancy tests during pandemic The Hospitalist
What is a risk evaluation and mitigation strategy (rems)? What is the ipledge rems? What is the ipledge program? To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special.
Fillable Online iPledge REMS Frequently Asked Questions (FAQ) Fax
To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge rems? The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required.
FDA Announces Modifications to iPLEDGE REMS Program MPR
What is a risk evaluation and mitigation strategy (rems)? A rems is a risk management program that uses risk. The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. What is the ipledge rems? To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has.
The Goals Of The Ipledge Rems Are To Prevent Fetal Exposure To Isotretinoin And To Inform Prescribers, Pharmacists, And Patients About.
What is the ipledge program? To avoid serious risks to unborn babies, the food and drug administration (fda) has required a special program called a risk evaluation and. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. What is a risk evaluation and mitigation strategy (rems)?
What Is The Ipledge Rems?
A rems is a risk management program that uses risk. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special.


![iPledge Login First Time at ️ [2023]](https://mytakesurvery.b-cdn.net/wp-content/uploads/2022/10/ipledge-login.jpg)





