Oxime Formation Mechanism
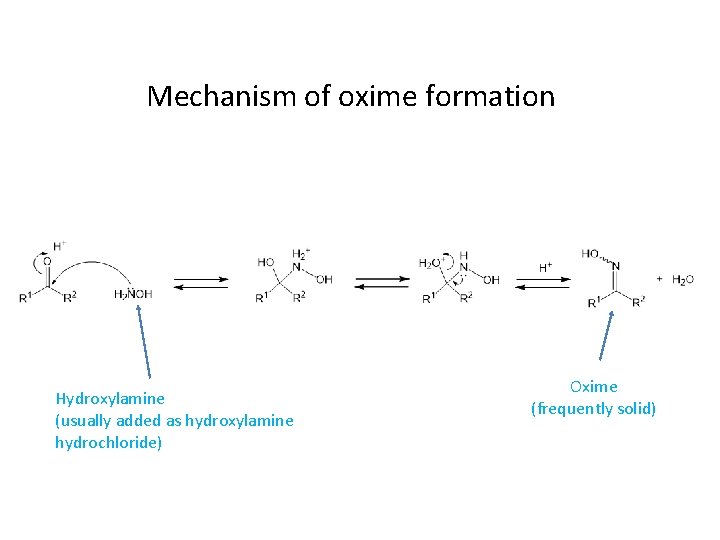
Oxime Formation Mechanism - The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. Reaction of aldehydes and ketones with hydroxylamine gives oximes. The nucleophilicity of the nitrogen on the hydroxylamine is.
The nucleophilicity of the nitrogen on the hydroxylamine is. The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. Reaction of aldehydes and ketones with hydroxylamine gives oximes.
The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. The nucleophilicity of the nitrogen on the hydroxylamine is. Reaction of aldehydes and ketones with hydroxylamine gives oximes.
Imines Properties, Formation, Reactions, and Mechanisms Master
The nucleophilicity of the nitrogen on the hydroxylamine is. The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. Reaction of aldehydes and ketones with hydroxylamine gives oximes.
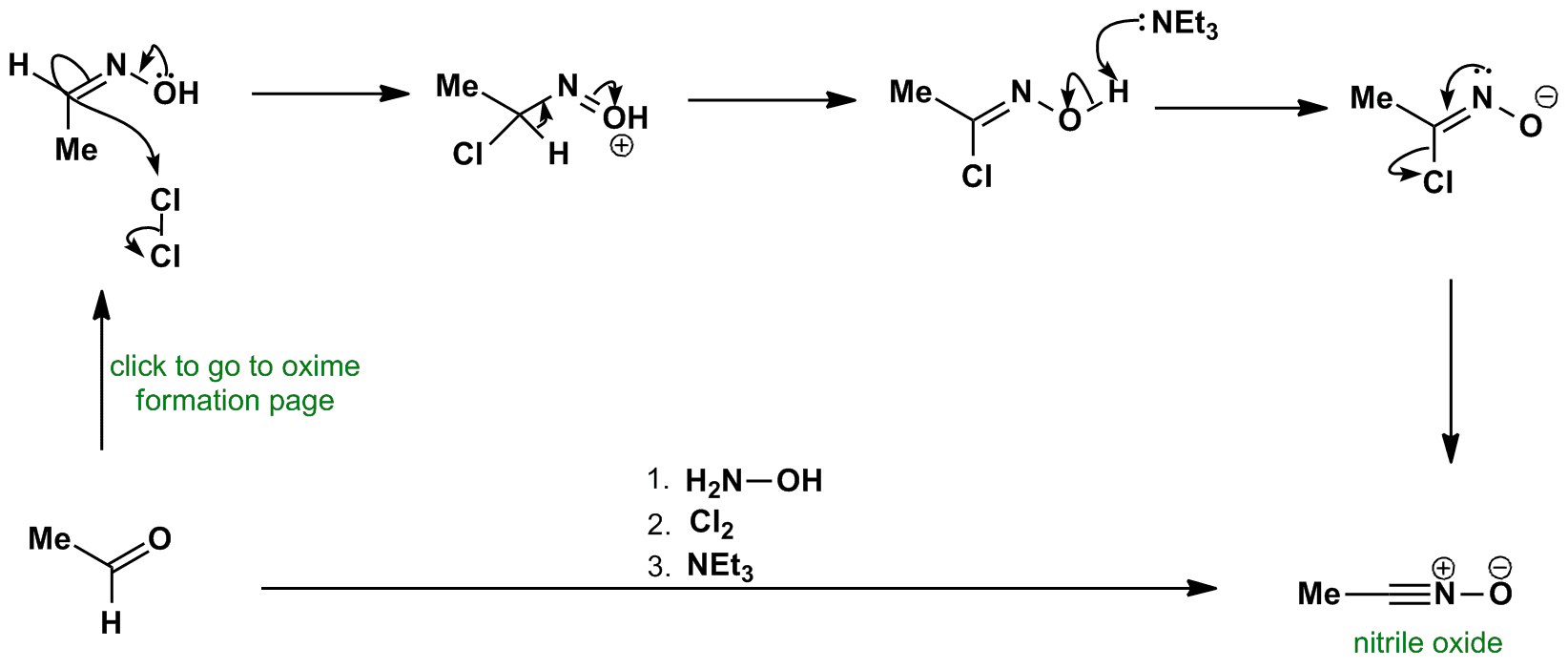
Nitrile Oxide Synthesis Via Oxime
The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. Reaction of aldehydes and ketones with hydroxylamine gives oximes. The nucleophilicity of the nitrogen on the hydroxylamine is.
Oxime Formation Step Optimization
The nucleophilicity of the nitrogen on the hydroxylamine is. The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. Reaction of aldehydes and ketones with hydroxylamine gives oximes.
Oxime
Reaction of aldehydes and ketones with hydroxylamine gives oximes. The nucleophilicity of the nitrogen on the hydroxylamine is. The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to.
[Solved] Q2. I need the oxime formation mechanism and the Beckmann
The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. The nucleophilicity of the nitrogen on the hydroxylamine is. Reaction of aldehydes and ketones with hydroxylamine gives oximes.
cyclohexanone oxime synthesis mechanism chemicalreaction YouTube
The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. Reaction of aldehydes and ketones with hydroxylamine gives oximes. The nucleophilicity of the nitrogen on the hydroxylamine is.
Proposed mechanistic pathway for the formation of the oximeoxime
The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. Reaction of aldehydes and ketones with hydroxylamine gives oximes. The nucleophilicity of the nitrogen on the hydroxylamine is.
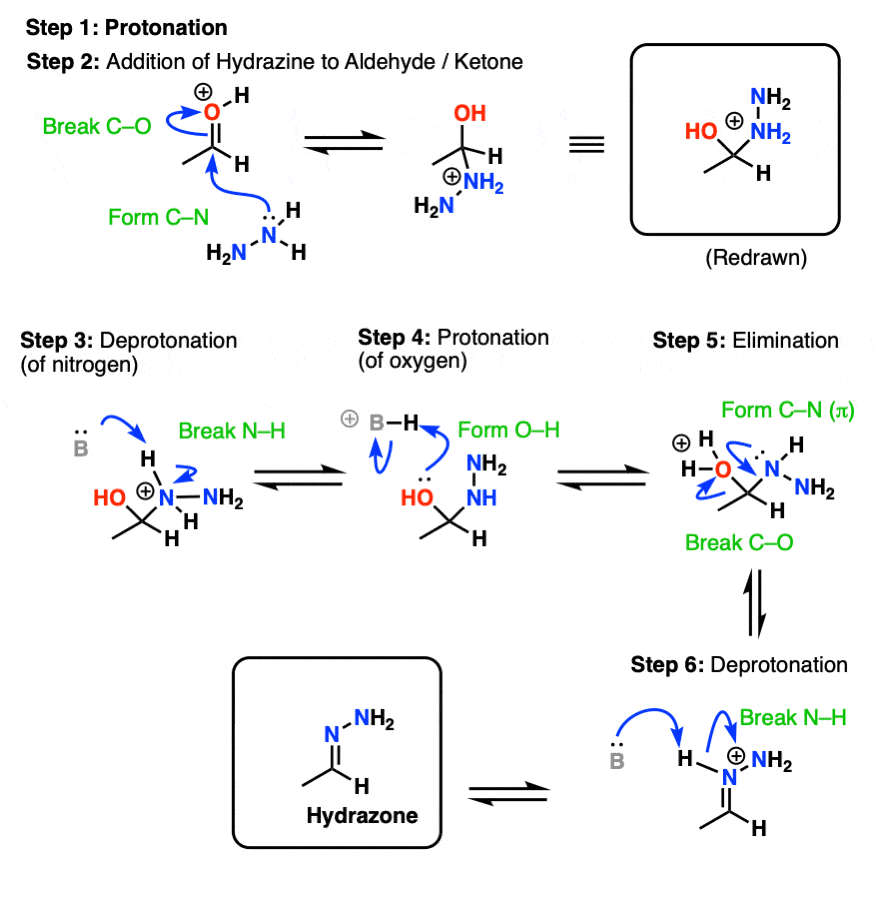
[PDF] Hydrolytic stability of hydrazones and oximes. Semantic Scholar
The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. Reaction of aldehydes and ketones with hydroxylamine gives oximes. The nucleophilicity of the nitrogen on the hydroxylamine is.
Formation of oximes and hydrazones Aldehydes and ketones Organic
The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to. The nucleophilicity of the nitrogen on the hydroxylamine is. Reaction of aldehydes and ketones with hydroxylamine gives oximes.
Other Reactions of Ketones and Aldehydes Relative Reactivity
Reaction of aldehydes and ketones with hydroxylamine gives oximes. The nucleophilicity of the nitrogen on the hydroxylamine is. The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to.
Reaction Of Aldehydes And Ketones With Hydroxylamine Gives Oximes.
The nucleophilicity of the nitrogen on the hydroxylamine is. The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to.




![[PDF] Hydrolytic stability of hydrazones and oximes. Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5dd85edc0941ee0d75a381fc4002f12644b2bc2f/10-Figure5-1.png)