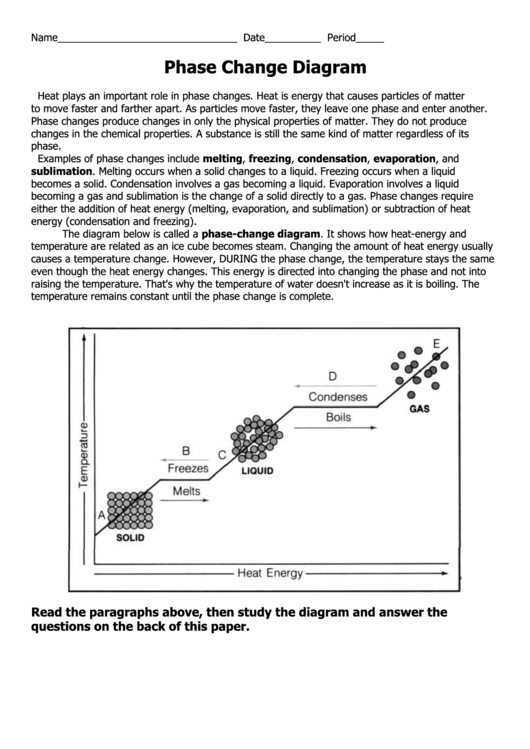
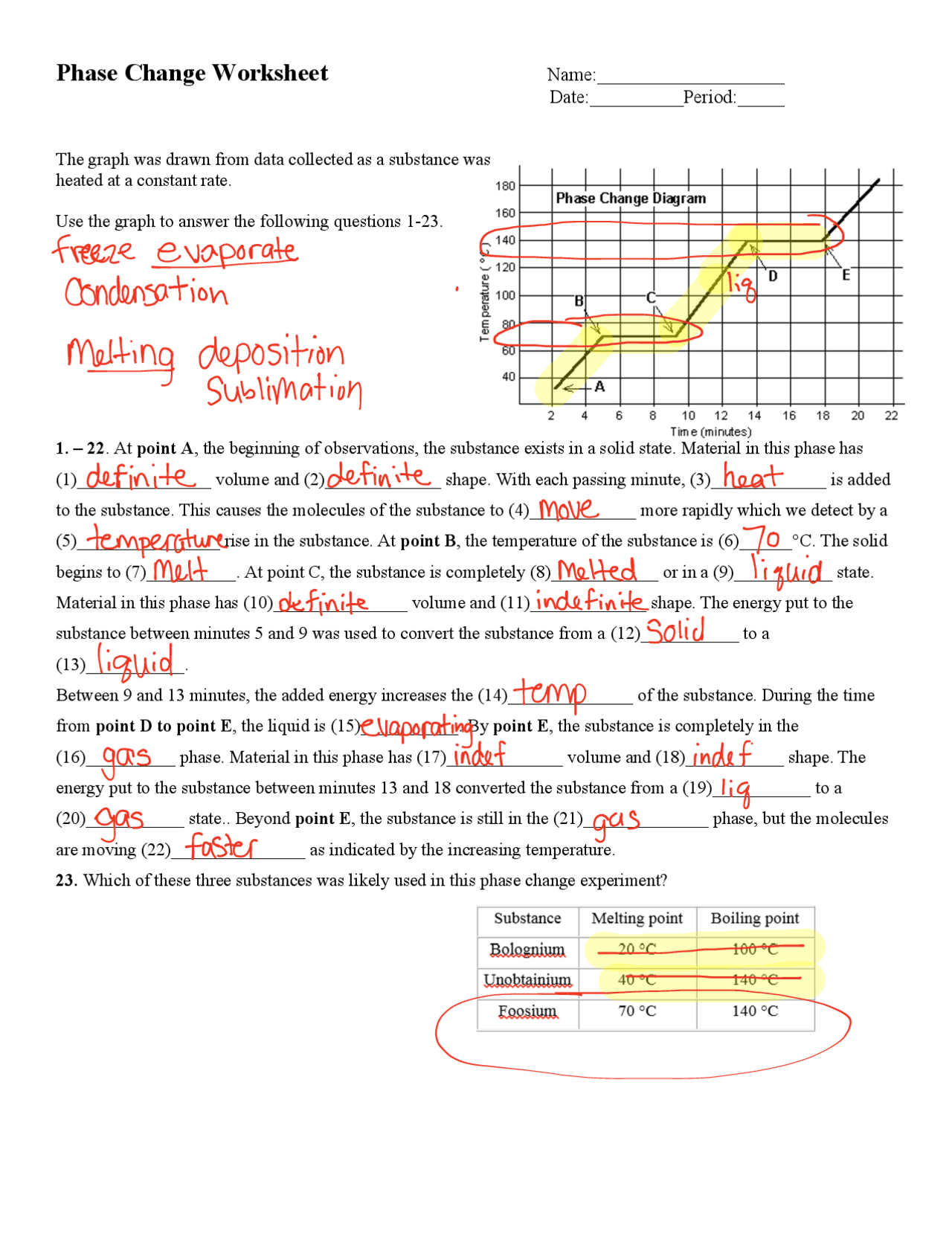
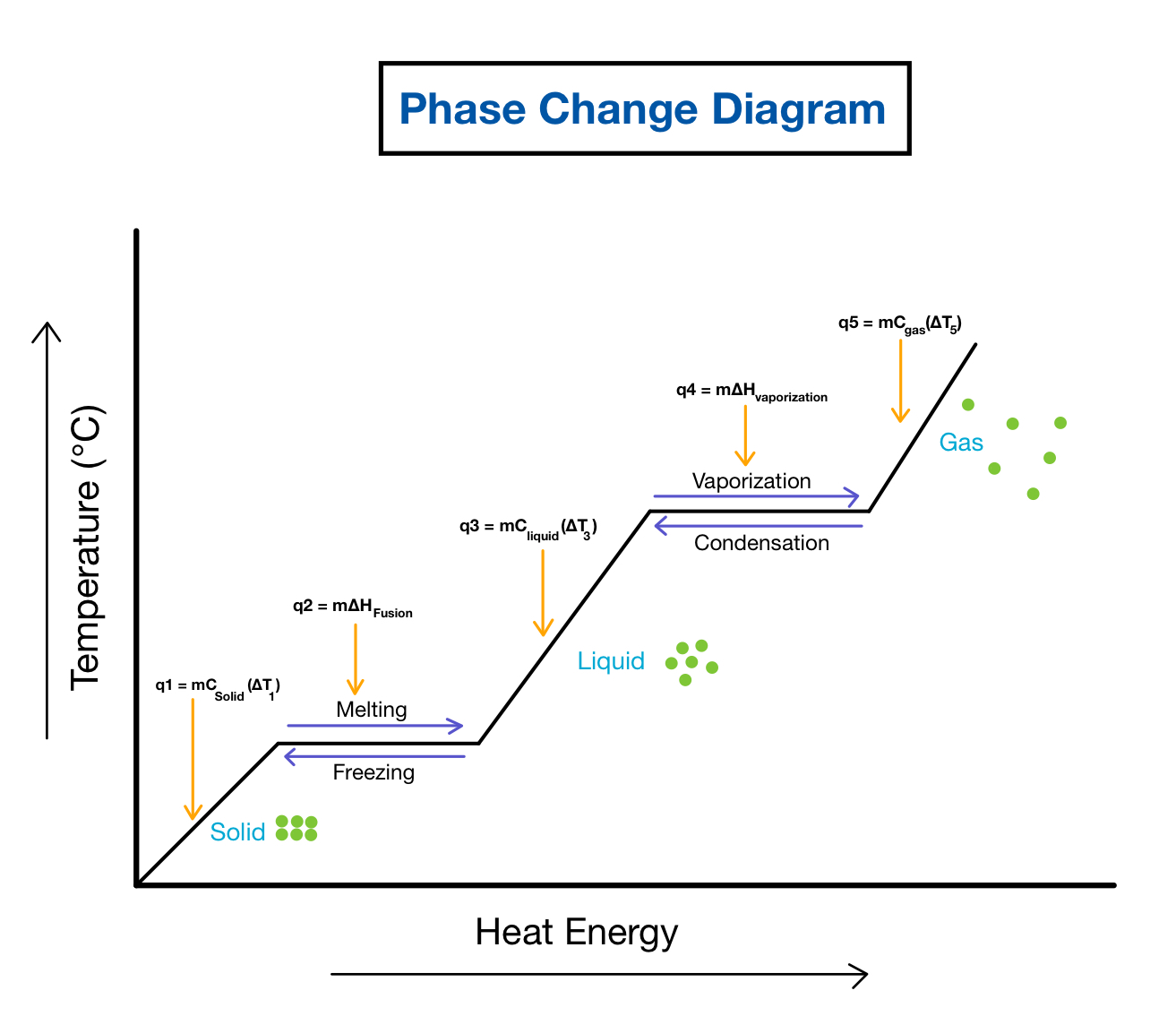
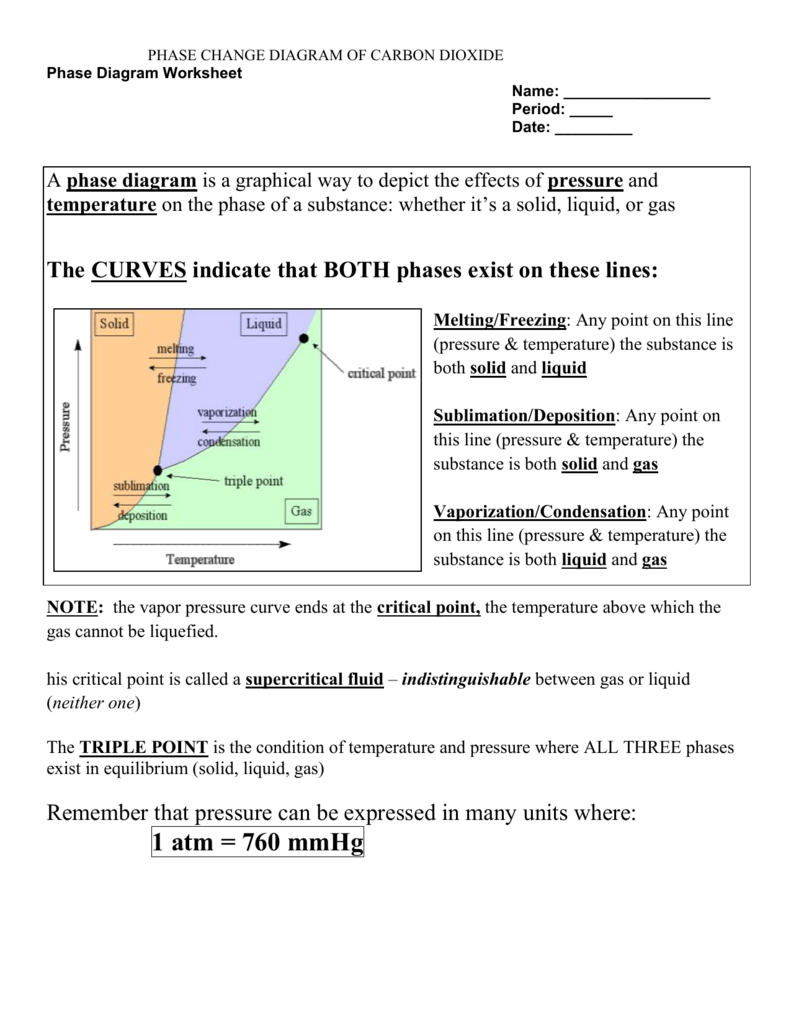
Phase Change Calculations Worksheet
Phase Change Calculations Worksheet - Heat with phase change worksheet 1) how many joules are required to heat 250 grams of liquid water from 0 0 to 100 0 c ? Calculations for temperature and phase change worksheet the heat of fusion of ice is 79.7 cal/g. What is the enthalpy of vaporization of a substance if it takes 34,212 j to boil away 25.0 g? Calculating heat of phase changes directions: Read and answer each of the following questions. Make sure to show all work, include. In this chemical change, something new (water) is formed, but a change of state (physical change) has also occured as a result. Specific heat, latent heat, phase change graphs, and calorimetry objective a: Caloric and joule’s discovery questions: The heat of vaporization of water is 540 cal/g.
Specific heat, latent heat, phase change graphs, and calorimetry objective a: Read and answer each of the following questions. Make sure to show all work, include. The heat of vaporization of water is 540 cal/g. Caloric and joule’s discovery questions: What is the enthalpy of vaporization of a substance if it takes 34,212 j to boil away 25.0 g? Heat with phase change worksheet 1) how many joules are required to heat 250 grams of liquid water from 0 0 to 100 0 c ? Calculating heat of phase changes directions: Calculations for temperature and phase change worksheet the heat of fusion of ice is 79.7 cal/g. In this chemical change, something new (water) is formed, but a change of state (physical change) has also occured as a result.
In this chemical change, something new (water) is formed, but a change of state (physical change) has also occured as a result. Heat with phase change worksheet 1) how many joules are required to heat 250 grams of liquid water from 0 0 to 100 0 c ? The heat of vaporization of water is 540 cal/g. What is the enthalpy of vaporization of a substance if it takes 34,212 j to boil away 25.0 g? Make sure to show all work, include. Caloric and joule’s discovery questions: Calculating heat of phase changes directions: Calculations for temperature and phase change worksheet the heat of fusion of ice is 79.7 cal/g. Specific heat, latent heat, phase change graphs, and calorimetry objective a: Read and answer each of the following questions.
Phase Change Diagram Science Worksheets printable pdf download
Specific heat, latent heat, phase change graphs, and calorimetry objective a: The heat of vaporization of water is 540 cal/g. Caloric and joule’s discovery questions: In this chemical change, something new (water) is formed, but a change of state (physical change) has also occured as a result. Calculations for temperature and phase change worksheet the heat of fusion of ice.
Phase Change Worksheet 1115479 Marie Anne Paule Live
Read and answer each of the following questions. Heat with phase change worksheet 1) how many joules are required to heat 250 grams of liquid water from 0 0 to 100 0 c ? The heat of vaporization of water is 540 cal/g. Caloric and joule’s discovery questions: What is the enthalpy of vaporization of a substance if it takes.
PS0203Phase Change Graph worksheet Live Worksheets Worksheets
The heat of vaporization of water is 540 cal/g. In this chemical change, something new (water) is formed, but a change of state (physical change) has also occured as a result. Calculating heat of phase changes directions: Specific heat, latent heat, phase change graphs, and calorimetry objective a: Read and answer each of the following questions.
Phase Change Worksheet with Answer Key Exercises Chemistry Docsity
Specific heat, latent heat, phase change graphs, and calorimetry objective a: In this chemical change, something new (water) is formed, but a change of state (physical change) has also occured as a result. What is the enthalpy of vaporization of a substance if it takes 34,212 j to boil away 25.0 g? Read and answer each of the following questions..
PS0201Phase Change Graph worksheet Live Worksheets Worksheets
Caloric and joule’s discovery questions: The heat of vaporization of water is 540 cal/g. Make sure to show all work, include. What is the enthalpy of vaporization of a substance if it takes 34,212 j to boil away 25.0 g? In this chemical change, something new (water) is formed, but a change of state (physical change) has also occured as.
Phase Change Diagram Worksheet
Specific heat, latent heat, phase change graphs, and calorimetry objective a: The heat of vaporization of water is 540 cal/g. Make sure to show all work, include. What is the enthalpy of vaporization of a substance if it takes 34,212 j to boil away 25.0 g? Caloric and joule’s discovery questions:
Phase Change Worksheet and Phase Change Diagram Studocu Worksheets
Calculating heat of phase changes directions: In this chemical change, something new (water) is formed, but a change of state (physical change) has also occured as a result. Make sure to show all work, include. What is the enthalpy of vaporization of a substance if it takes 34,212 j to boil away 25.0 g? Caloric and joule’s discovery questions:
Phase Change Diagrams — Overview & Examples Expii
Heat with phase change worksheet 1) how many joules are required to heat 250 grams of liquid water from 0 0 to 100 0 c ? The heat of vaporization of water is 540 cal/g. What is the enthalpy of vaporization of a substance if it takes 34,212 j to boil away 25.0 g? Calculations for temperature and phase change.
Phase change calculations YouTube
The heat of vaporization of water is 540 cal/g. Specific heat, latent heat, phase change graphs, and calorimetry objective a: Make sure to show all work, include. What is the enthalpy of vaporization of a substance if it takes 34,212 j to boil away 25.0 g? Read and answer each of the following questions.
Phase Change Worksheet Answers Englishworksheet.my.id
Calculations for temperature and phase change worksheet the heat of fusion of ice is 79.7 cal/g. In this chemical change, something new (water) is formed, but a change of state (physical change) has also occured as a result. Calculating heat of phase changes directions: The heat of vaporization of water is 540 cal/g. Make sure to show all work, include.
In This Chemical Change, Something New (Water) Is Formed, But A Change Of State (Physical Change) Has Also Occured As A Result.
Heat with phase change worksheet 1) how many joules are required to heat 250 grams of liquid water from 0 0 to 100 0 c ? Specific heat, latent heat, phase change graphs, and calorimetry objective a: What is the enthalpy of vaporization of a substance if it takes 34,212 j to boil away 25.0 g? Make sure to show all work, include.
Caloric And Joule’s Discovery Questions:
Calculations for temperature and phase change worksheet the heat of fusion of ice is 79.7 cal/g. Calculating heat of phase changes directions: Read and answer each of the following questions. The heat of vaporization of water is 540 cal/g.