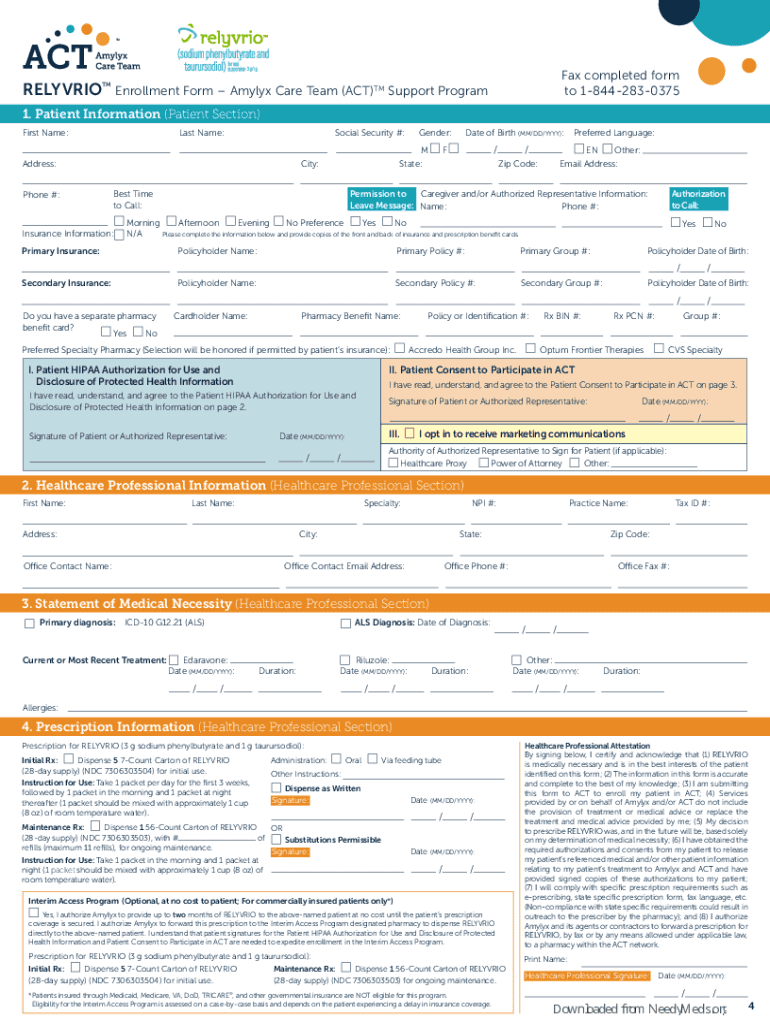
Relyvrio Enrollment Form
Relyvrio Enrollment Form - In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Please sign section ii on the enrollment form to document your agreement to this patient consent to participate in act. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. The act support program provides support to eligible patients with als who have been prescribed relyvrio (sodium phenylbutyrate and. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. Discuss the process, understand how to submit. If you and your doctor determine that relyvrio is right for you, your doctor will complete the relyvrio enrollment form with you, which enrolls you in the.
Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. The act support program provides support to eligible patients with als who have been prescribed relyvrio (sodium phenylbutyrate and. Please sign section ii on the enrollment form to document your agreement to this patient consent to participate in act. Discuss the process, understand how to submit. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. If you and your doctor determine that relyvrio is right for you, your doctor will complete the relyvrio enrollment form with you, which enrolls you in the.
Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. If you and your doctor determine that relyvrio is right for you, your doctor will complete the relyvrio enrollment form with you, which enrolls you in the. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. The act support program provides support to eligible patients with als who have been prescribed relyvrio (sodium phenylbutyrate and. Discuss the process, understand how to submit. Please sign section ii on the enrollment form to document your agreement to this patient consent to participate in act.
FDA Approves AMX0035 (Relyvrio) for ALS
If you and your doctor determine that relyvrio is right for you, your doctor will complete the relyvrio enrollment form with you, which enrolls you in the. The act support program provides support to eligible patients with als who have been prescribed relyvrio (sodium phenylbutyrate and. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support.
Fillable Online RELYVRIO Enrollment Form Amylyx Care Team (ACT) Support
Discuss the process, understand how to submit. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. The act support program provides support to eligible patients with als who have been prescribed relyvrio (sodium phenylbutyrate and. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. If.
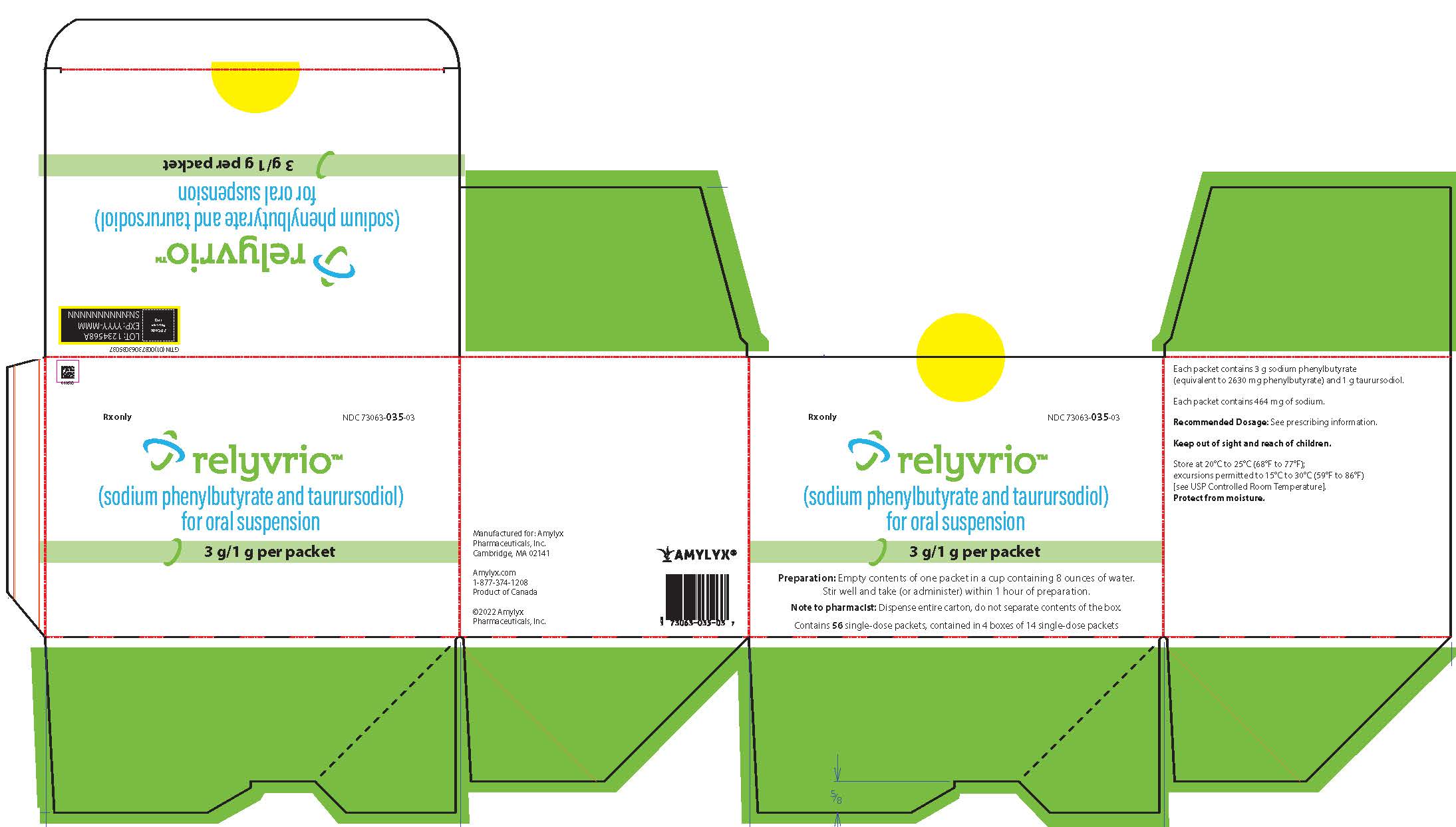
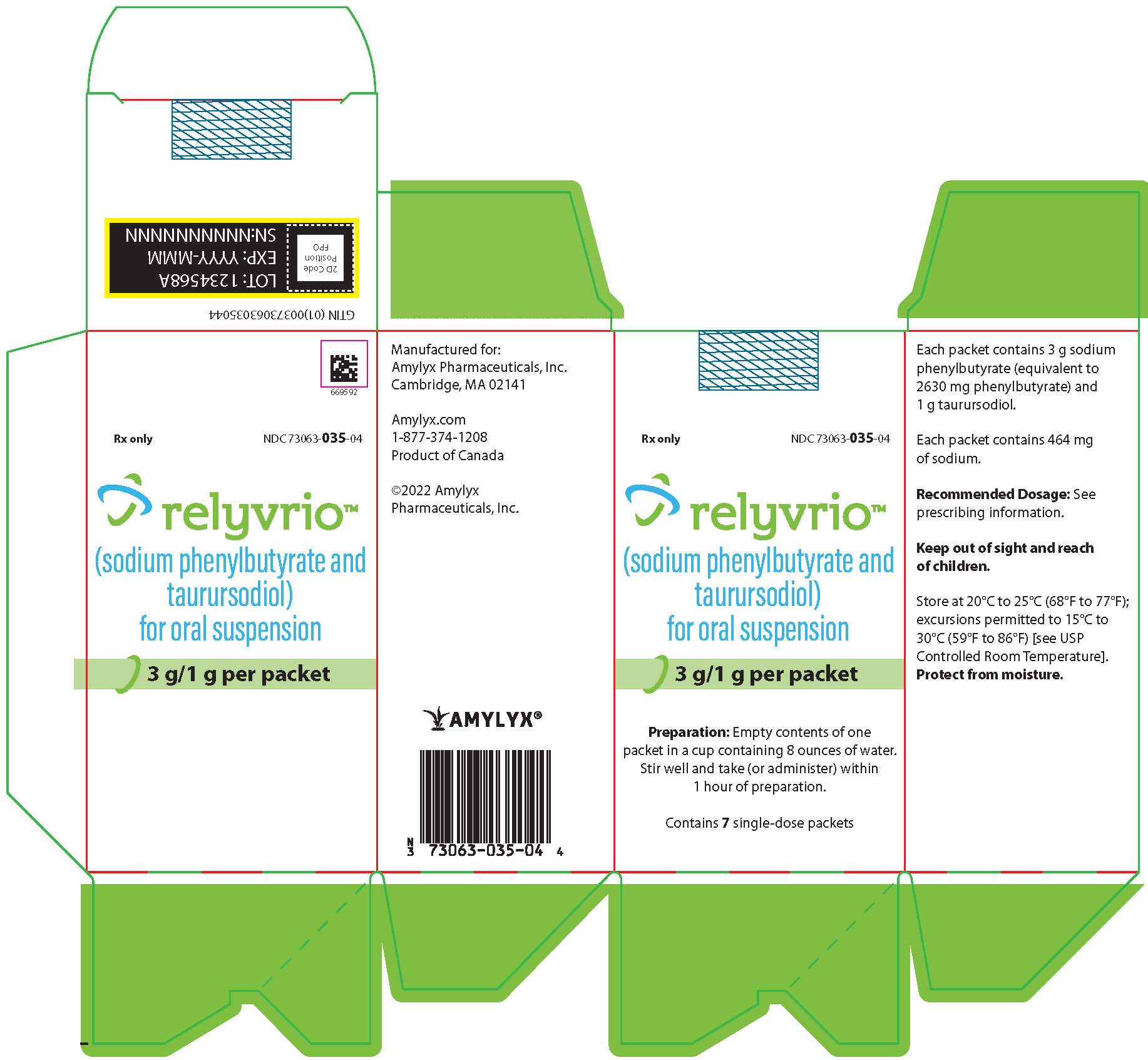
DailyMed RELYVRIO sodium phenylbutyrate/taurursodiol powder, for
The act support program provides support to eligible patients with als who have been prescribed relyvrio (sodium phenylbutyrate and. If you and your doctor determine that relyvrio is right for you, your doctor will complete the relyvrio enrollment form with you, which enrolls you in the. Please sign section ii on the enrollment form to document your agreement to this.
October 2022 Foundation eNews Les Turner ALS Foundation
If you and your doctor determine that relyvrio is right for you, your doctor will complete the relyvrio enrollment form with you, which enrolls you in the. The act support program provides support to eligible patients with als who have been prescribed relyvrio (sodium phenylbutyrate and. Please sign section ii on the enrollment form to document your agreement to this.
200年上市4款药,“渐冻人”何时能解冻?—新闻—科学网
Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. Discuss the process, understand how to submit. The act support program provides support to eligible patients with als who have been prescribed relyvrio (sodium.
Relyvrio, el medicamento aprobado por la FDA para combatir la
Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. If you and your doctor determine that relyvrio is right for you, your doctor will complete the relyvrio enrollment form with you, which enrolls you in the. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023.
RELYVRIO sodium phenylbutyrate/taurursodiol powder, for suspension
Please sign section ii on the enrollment form to document your agreement to this patient consent to participate in act. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. The.
Newly Approved ALS Drug Relyvrio To Face Its Next Challenge Pricing
The act support program provides support to eligible patients with als who have been prescribed relyvrio (sodium phenylbutyrate and. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Discuss the.
Fillable Online RELYVRIO Enrollment Form Guide. RELYVRIO Enrollment
The act support program provides support to eligible patients with als who have been prescribed relyvrio (sodium phenylbutyrate and. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. If you and your doctor determine that relyvrio is right for you, your doctor will complete the relyvrio enrollment form with you, which enrolls you in.
FDA Approves New ALS Drug Relyvrio That Aims to Slow Disease Progression
Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in. Medication relyvrio ® (sodium phenylbutyrate and taurursodiol) p&t approval date 12/2022, 12/2023 effective date 3/1/2024. Please sign section ii on the enrollment form to document your agreement to this patient consent to participate in act. If you and your.
Medication Relyvrio ® (Sodium Phenylbutyrate And Taurursodiol) P&T Approval Date 12/2022, 12/2023 Effective Date 3/1/2024.
Please sign section ii on the enrollment form to document your agreement to this patient consent to participate in act. In order to submit a javygtor prescription, the prescribing provider will need to complete a therapy enrollment form. Discuss the process, understand how to submit. Albrioza is now available only to current patients under amylyx pharmaceuticals, inc.’s patient support program and should not be initiated in.
The Act Support Program Provides Support To Eligible Patients With Als Who Have Been Prescribed Relyvrio (Sodium Phenylbutyrate And.
If you and your doctor determine that relyvrio is right for you, your doctor will complete the relyvrio enrollment form with you, which enrolls you in the.





:max_bytes(150000):strip_icc()/relyvrio-credit-Amylyx-Pharmaceuticals-d5ab86a7fdf84d65b8f54eed10a94ee8.jpg)