Research Consent Form Example
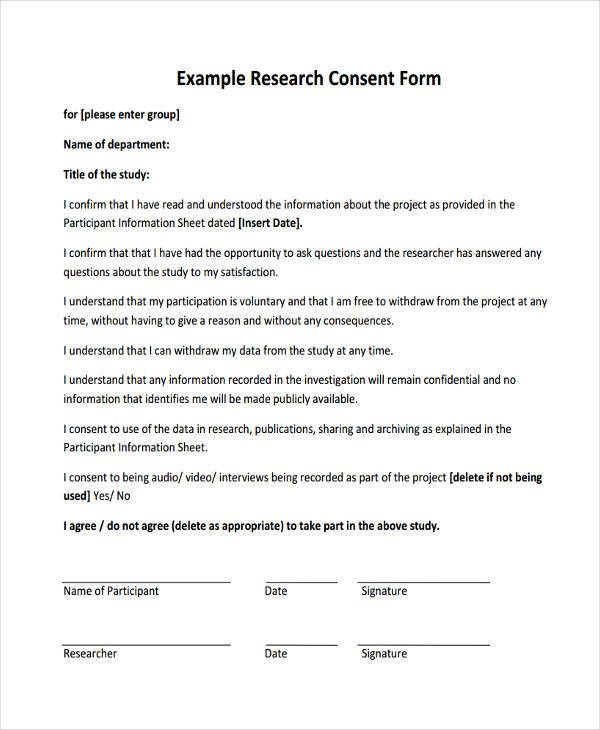
Research Consent Form Example - When completing and irb submission in irbis, please fill in the. View the sample forms listed. These consent form templates have been posted for your reference. • use a file name(s) that clearly identify each consent document (e.g. Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. This effort can be lessened by. These new templates should be used for any new consent form being submitted to the irb. General consent form to participate in research (doc) two stage project consent form (doc) parent permission form for research with child (doc)
This effort can be lessened by. Online consent, parental permission, adult consent, teacher consent,. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. General consent form to participate in research (doc) two stage project consent form (doc) parent permission form for research with child (doc) A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. • use a file name(s) that clearly identify each consent document (e.g. Here are also examples of the concise summary that must. When completing and irb submission in irbis, please fill in the. These new templates should be used for any new consent form being submitted to the irb.
General consent form to participate in research (doc) two stage project consent form (doc) parent permission form for research with child (doc) A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. Online consent, parental permission, adult consent, teacher consent,. These new templates should be used for any new consent form being submitted to the irb. These consent form templates have been posted for your reference. View the sample forms listed. Here are also examples of the concise summary that must. • use a file name(s) that clearly identify each consent document (e.g. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort.
FREE 12+ Research Consent Form Samples, PDF, MS Word, Google Docs
View the sample forms listed. Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. • use a file name(s) that clearly identify each consent document (e.g. This effort can be lessened by. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort.
FREE 8+ Research Informed Consent Forms in PDF MS Word
• use a file name(s) that clearly identify each consent document (e.g. These new templates should be used for any new consent form being submitted to the irb. Here are also examples of the concise summary that must. Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. Online consent, parental permission,.
Dissertation Data Collection Consent Form Example
Online consent, parental permission, adult consent, teacher consent,. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. These new templates should be used for any new consent form being submitted to the irb. Here are also examples of the concise summary that must. This effort can be lessened by.
FREE 12+ Research Consent Form Samples & Templates
General consent form to participate in research (doc) two stage project consent form (doc) parent permission form for research with child (doc) • use a file name(s) that clearly identify each consent document (e.g. When completing and irb submission in irbis, please fill in the. Here are also examples of the concise summary that must. These consent form templates have.
FREE 12+ Research Consent Form Samples, PDF, MS Word, Google Docs
• use a file name(s) that clearly identify each consent document (e.g. Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. This effort can be lessened by. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. View the sample forms listed.
Sample Research Consent Form 8+ Free Documents Download in PDF, Word
These new templates should be used for any new consent form being submitted to the irb. These consent form templates have been posted for your reference. Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. View the sample forms listed. General consent form to participate in research (doc) two stage project.
FREE 6+ Research Consent Forms in PDF MS Word
Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. • use a file name(s) that clearly identify each consent document (e.g. Here are also examples of the concise summary that must. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. These consent form.
Consent Form For Interview Research Printable Consent Form
Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. • use a file name(s) that clearly identify each consent document (e.g. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. These consent form templates have been posted for your reference. Online.
FREE 12+ Research Consent Form Samples & Templates
Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. View the sample forms listed. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. These.
Free Research Informed Consent Form PDF Word eForms
Here are also examples of the concise summary that must. This effort can be lessened by. Consent forms should include information about the investigator, the topic, and a description of purpose, risks, and benefits. Online consent, parental permission, adult consent, teacher consent,. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort.
Consent Forms Should Include Information About The Investigator, The Topic, And A Description Of Purpose, Risks, And Benefits.
View the sample forms listed. General consent form to participate in research (doc) two stage project consent form (doc) parent permission form for research with child (doc) This effort can be lessened by. Here are also examples of the concise summary that must.
Writing A Consent Form That Uses Plain Language, And That Is Brief And Clear, Requires Substantial Effort.
• use a file name(s) that clearly identify each consent document (e.g. When completing and irb submission in irbis, please fill in the. These new templates should be used for any new consent form being submitted to the irb. Online consent, parental permission, adult consent, teacher consent,.
These Consent Form Templates Have Been Posted For Your Reference.
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.