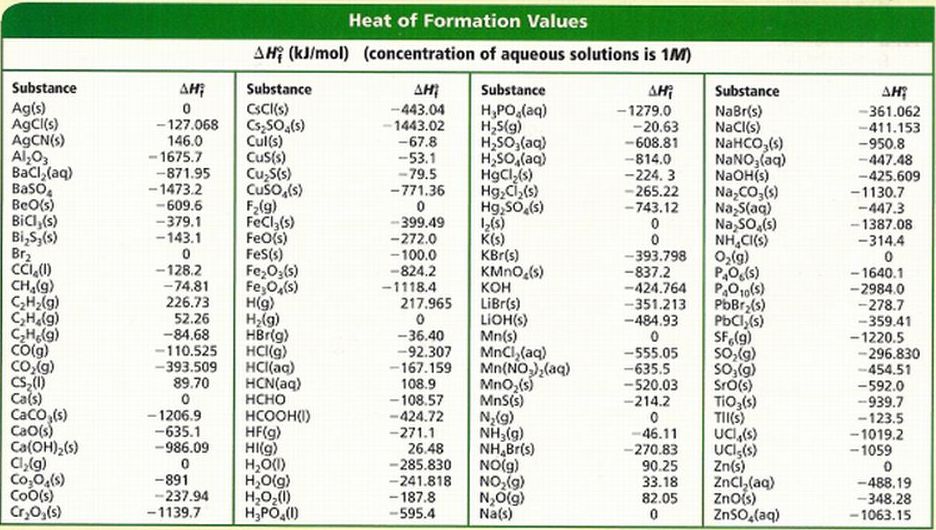
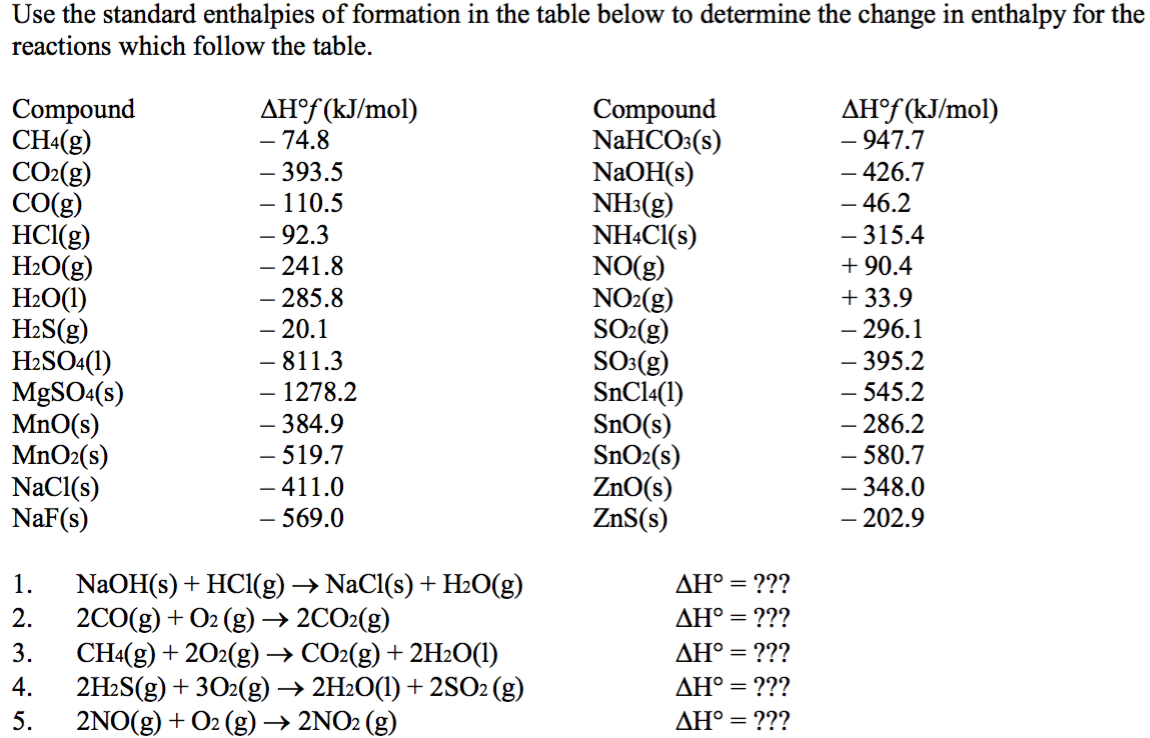
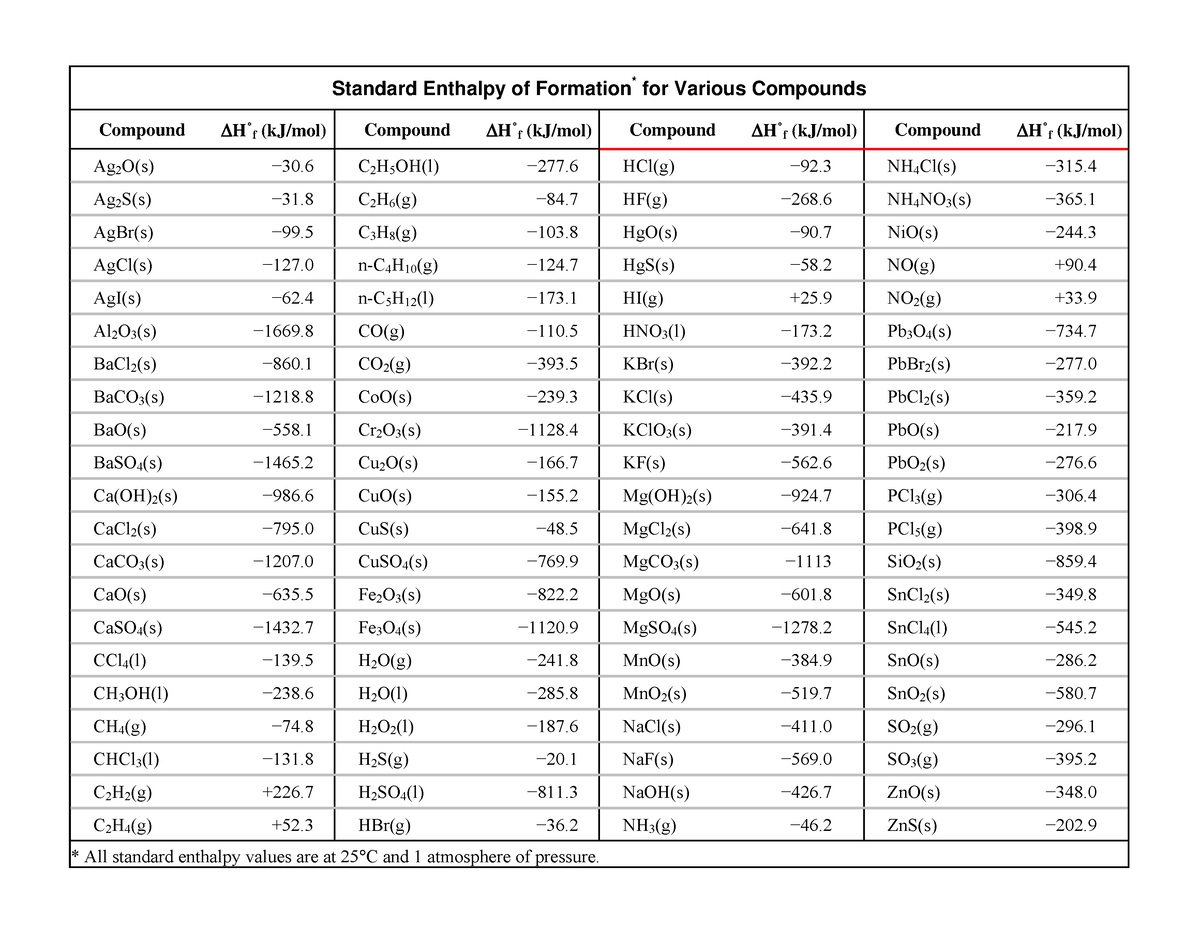
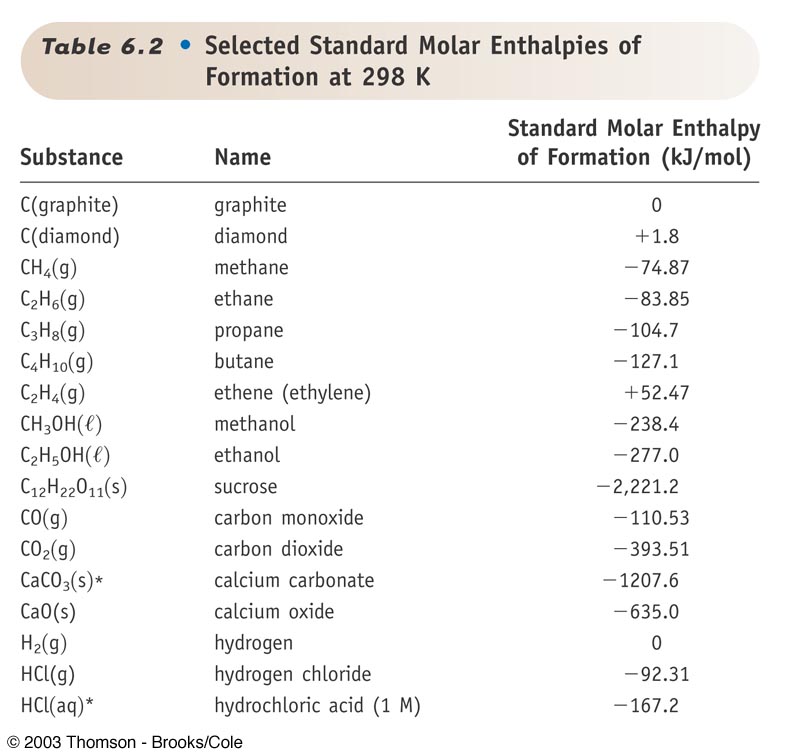
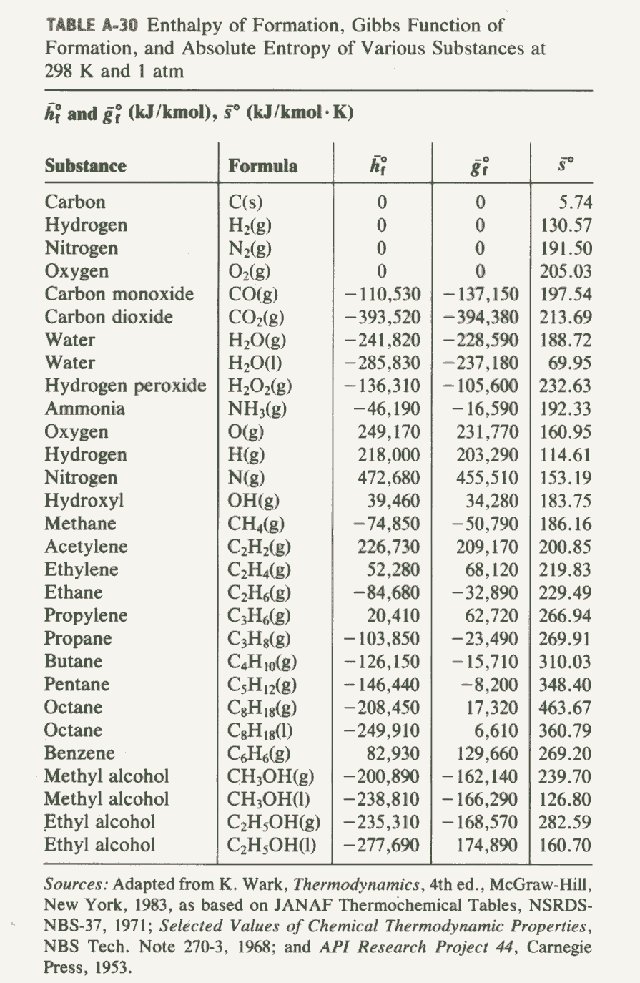
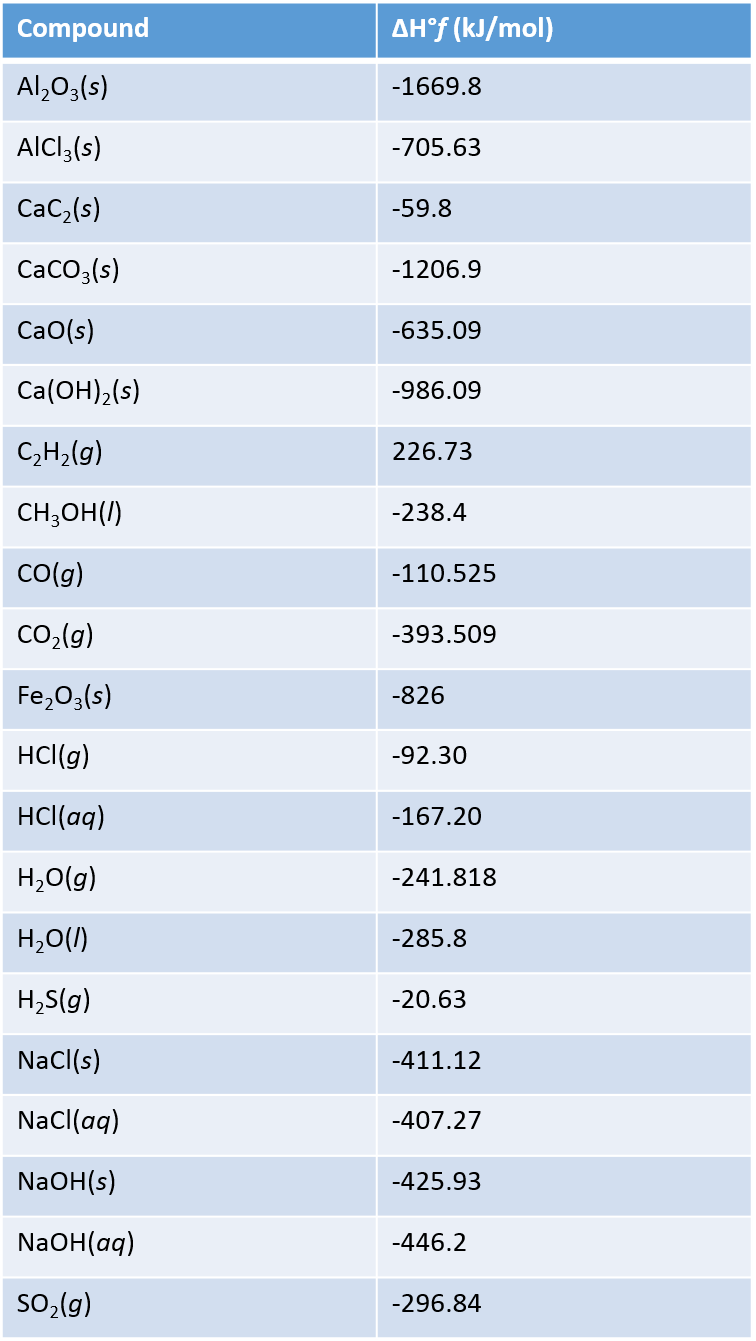
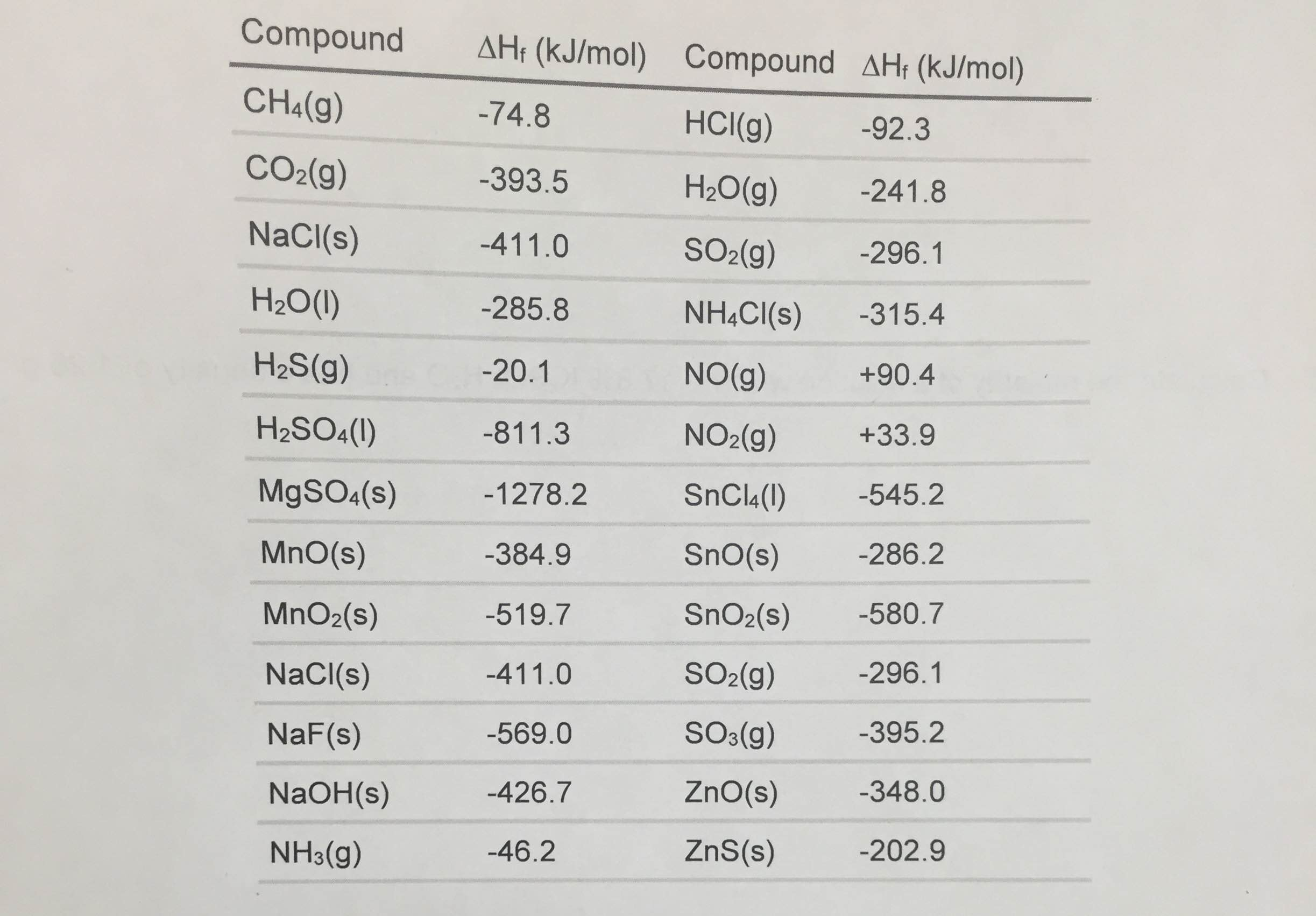
Standard Enthalpy Formation Table
Standard Enthalpy Formation Table - This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. ∗the standard entropy of the h+(aq) ion is defined to be 0.
∗the standard entropy of the h+(aq) ion is defined to be 0. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure.
* all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. ∗the standard entropy of the h+(aq) ion is defined to be 0. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,.
III Calculating Enthalpies STA Form IV Honors Chemistry
∗the standard entropy of the h+(aq) ion is defined to be 0. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. This table lists the standard enthalpies (δh°), the free energies (δg°).
Solved Use the standard enthalpies of formation in the table
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and.
Change in Enthalpy Chemistry lessons, Chemistry, Ap chemistry
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. ∗the standard entropy of the h+(aq) ion is defined to be 0. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The table below shows the standard enthalpy of formation, the.
Standard Enthalpy of Formation Table Standard Enthalpy of Formation
∗the standard entropy of the h+(aq) ion is defined to be 0. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. * all standard enthalpy.
Heat Of Formation Chart
The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements.
Standard enthalpy of formation and standard free energy of formation of
∗the standard entropy of the h+(aq) ion is defined to be 0. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The table below shows the standard enthalpy of formation, the.
Enthalpies of formation for stable and radical species used in work
* all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. ∗the standard entropy of the h+(aq) ion is defined to be 0. The table below shows the standard enthalpy of formation, the.
Standard Enthalpies of Formation SchoolWorkHelper
* all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. ∗the standard entropy of the h+(aq) ion is defined to be 0. The table below shows the standard enthalpy of formation, the.
Solved Using the table of standard enthalpies of formation
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. ∗the standard entropy of the h+(aq) ion is defined to be 0. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. This table lists the standard enthalpies (δh°), the free energies.
Solved Use a standard enthalpies of formation table to
∗the standard entropy of the h+(aq) ion is defined to be 0. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The table below shows the standard enthalpy of formation, the.
This Table Lists The Standard Enthalpies (Δh°), The Free Energies (Δg°) Of Formation Of Compounds From Elements In Their Standard States, And The.
* all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. ∗the standard entropy of the h+(aq) ion is defined to be 0.