What Does The Atomic Number Of An Element Specify
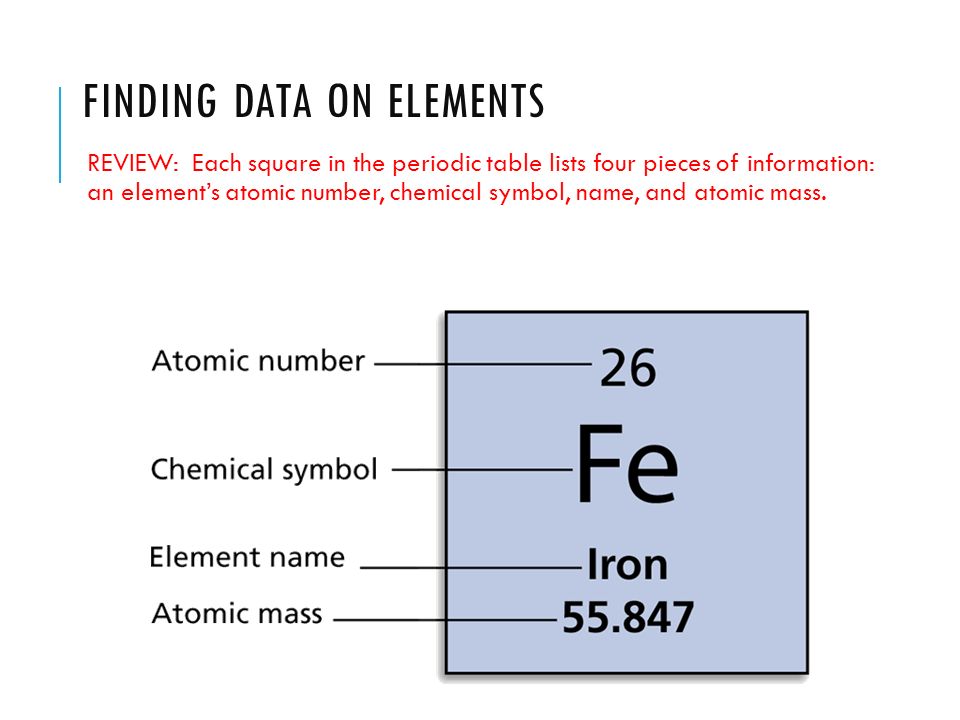
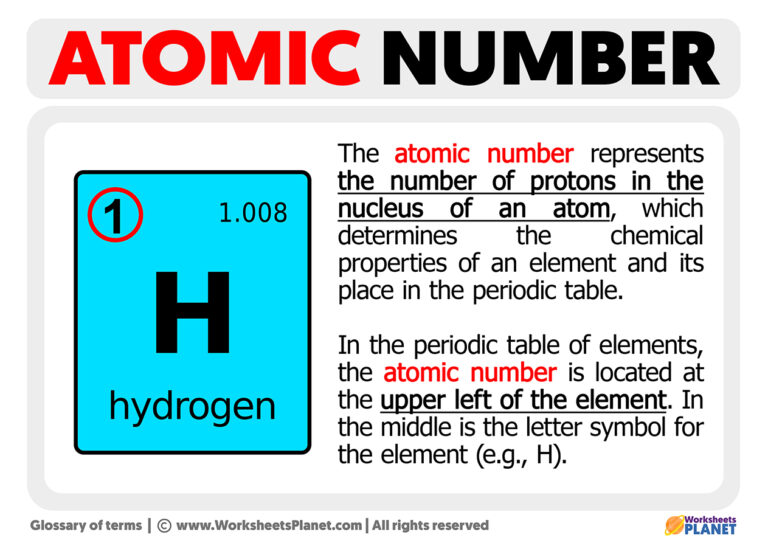
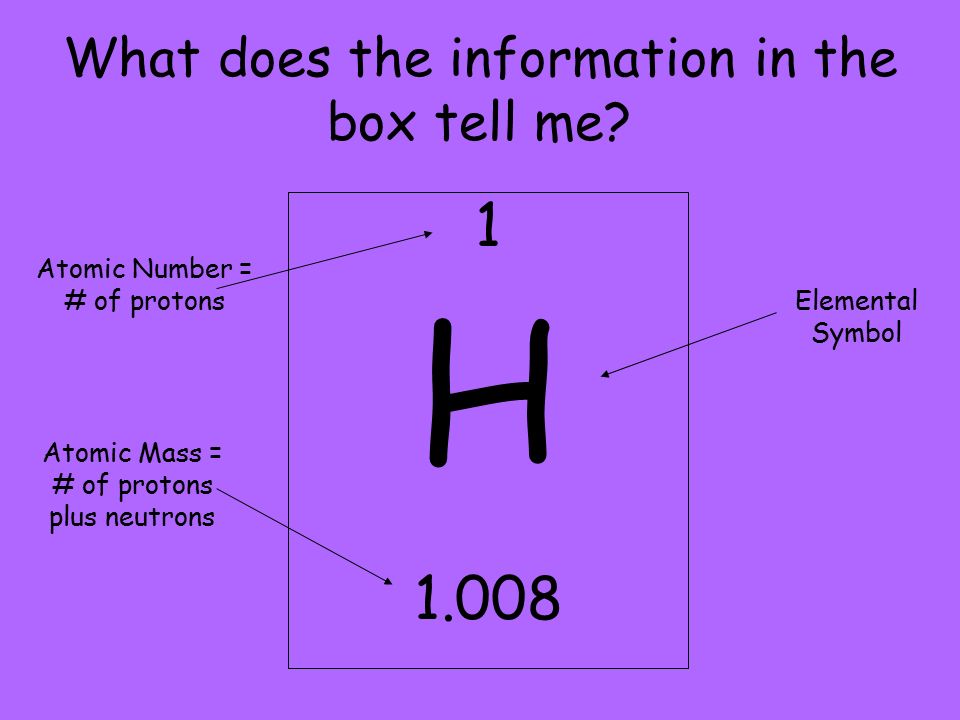
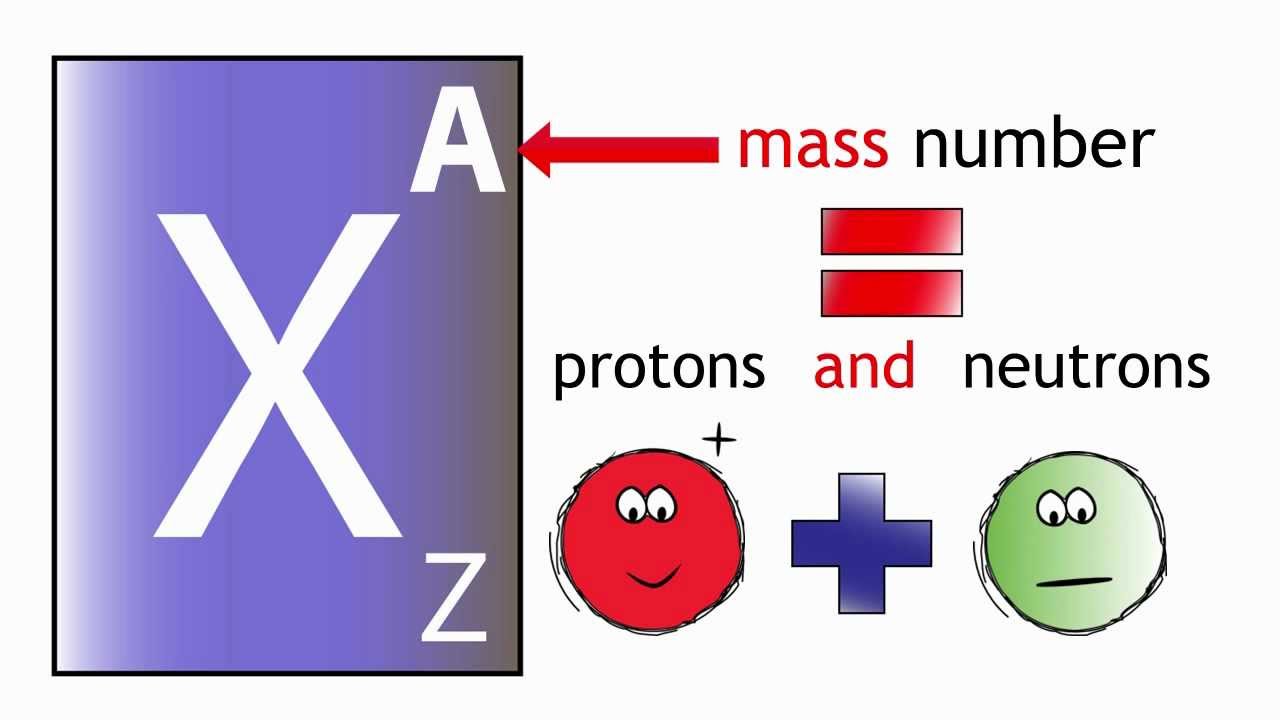
What Does The Atomic Number Of An Element Specify - The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. Thus, hydrogen has an atomic number of 1, while iron has an. The number of protons in an atom is the atomic number of the element. This means that the number of.
The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. This means that the number of. The number of protons in an atom is the atomic number of the element. Thus, hydrogen has an atomic number of 1, while iron has an.
The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. Thus, hydrogen has an atomic number of 1, while iron has an. This means that the number of. The number of protons in an atom is the atomic number of the element.
What is an element?
The number of protons in an atom is the atomic number of the element. This means that the number of. Thus, hydrogen has an atomic number of 1, while iron has an. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element.
Atomic Number and Mass Number ALevel Chemistry
Thus, hydrogen has an atomic number of 1, while iron has an. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. This means that the number of. The number of protons in an atom is the atomic number of the element.
Properties of Elements Biology for NonMajors I
The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. The number of protons in an atom is the atomic number of the element. This means that the number of. Thus, hydrogen has an atomic number of 1, while iron has an.
What is the Atomic Number? Worksheets
Thus, hydrogen has an atomic number of 1, while iron has an. This means that the number of. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. The number of protons in an atom is the atomic number of the element.
🤔What Does The Atomic Number Represent🤔
The number of protons in an atom is the atomic number of the element. Thus, hydrogen has an atomic number of 1, while iron has an. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. This means that the number of.
List of Elements By Atomic Number
The number of protons in an atom is the atomic number of the element. This means that the number of. Thus, hydrogen has an atomic number of 1, while iron has an. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element.
How To Find Element Atomic Number, Element Name & Symbol
The number of protons in an atom is the atomic number of the element. This means that the number of. Thus, hydrogen has an atomic number of 1, while iron has an. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element.
What Element Contains The Name Of Another Element Within It Hot Sale
Thus, hydrogen has an atomic number of 1, while iron has an. This means that the number of. The number of protons in an atom is the atomic number of the element. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element.
what is the atomic number
This means that the number of. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. Thus, hydrogen has an atomic number of 1, while iron has an. The number of protons in an atom is the atomic number of the element.
How To Find Element Atomic Number, Element Name & Symbol
This means that the number of. Thus, hydrogen has an atomic number of 1, while iron has an. The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. The number of protons in an atom is the atomic number of the element.
The Number Of Protons In An Atom Is The Atomic Number Of The Element.
The atomic number (z) of an element is the number of protons in the nucleus of each atom of that element. Thus, hydrogen has an atomic number of 1, while iron has an. This means that the number of.
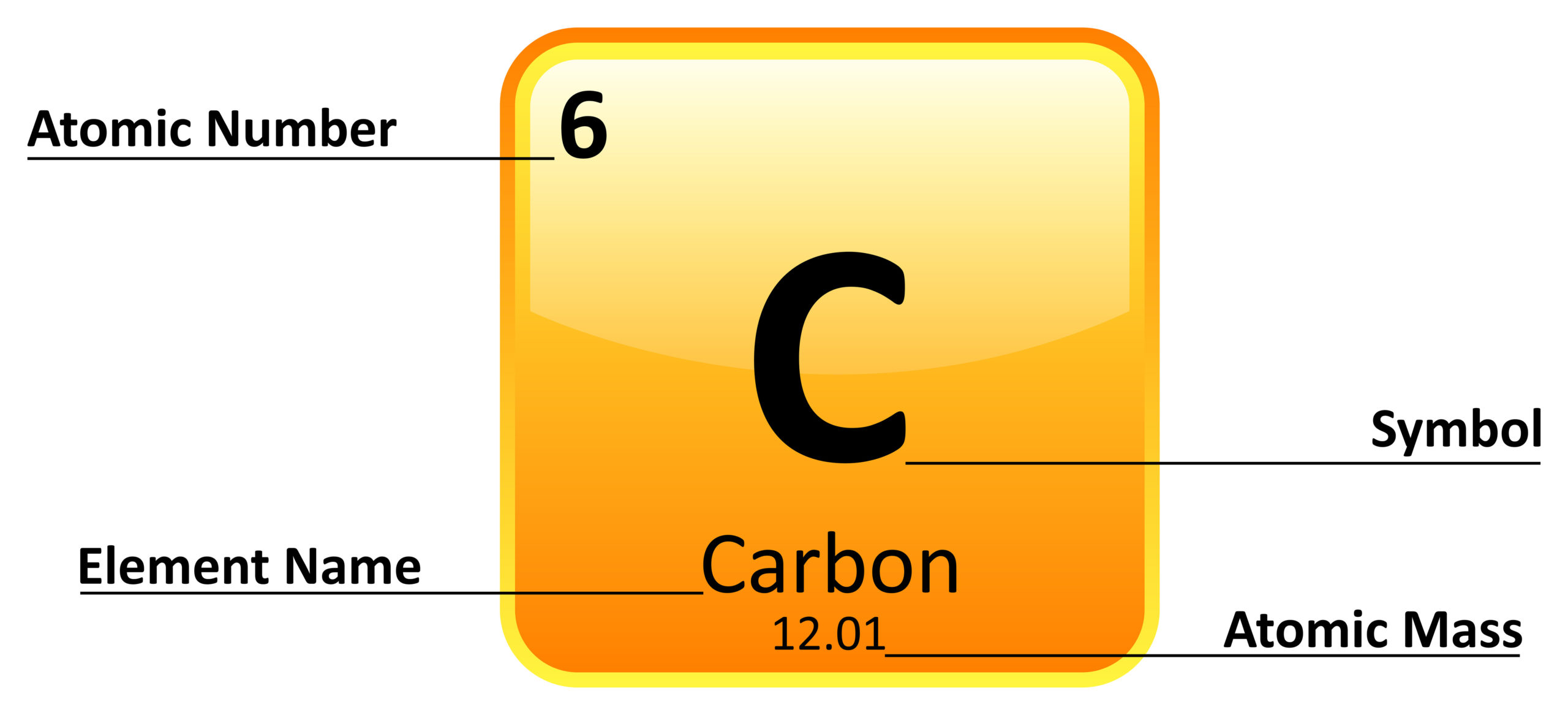
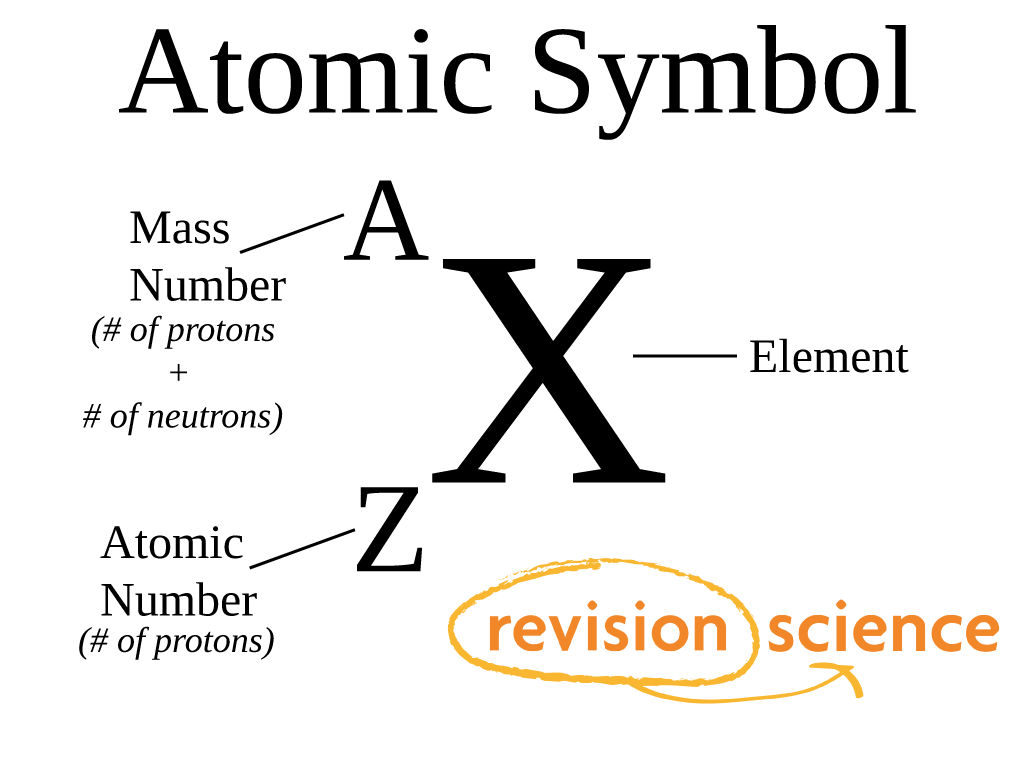
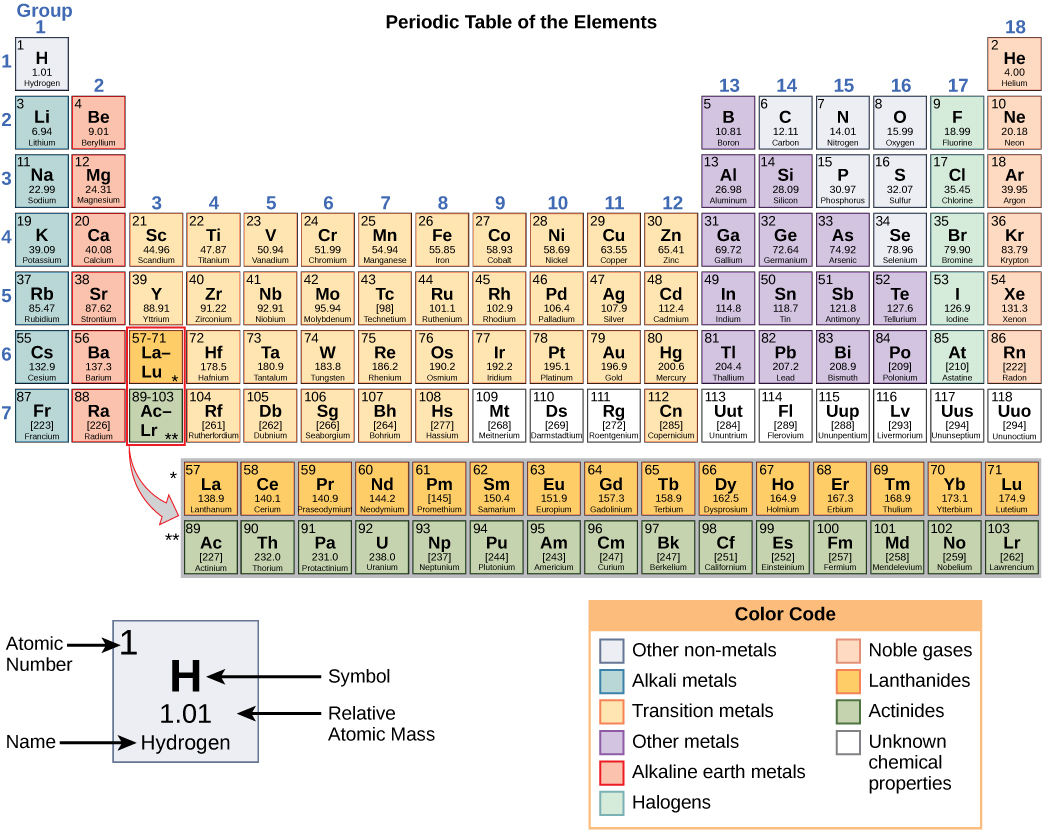

:max_bytes(150000):strip_icc()/element-list-names-atomic-numbers-606529_V1_FINAL-f332cfc84a494b7782d84fc986cdaf86.png)
.PNG)