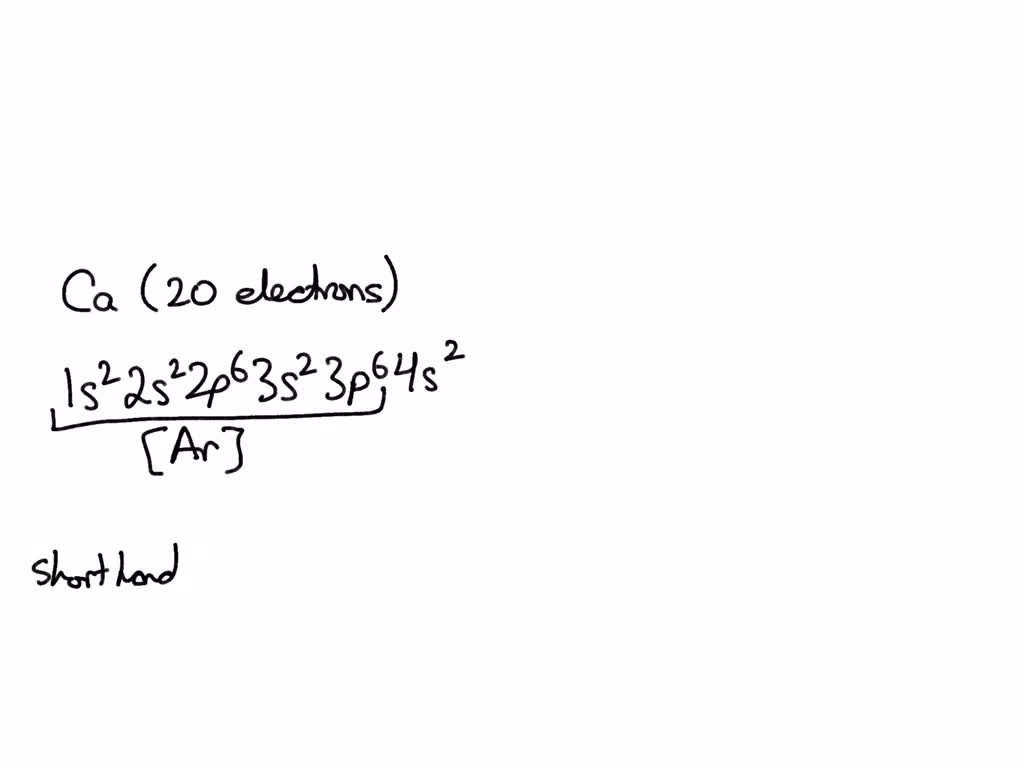What Is The Electron Configuration For Ca
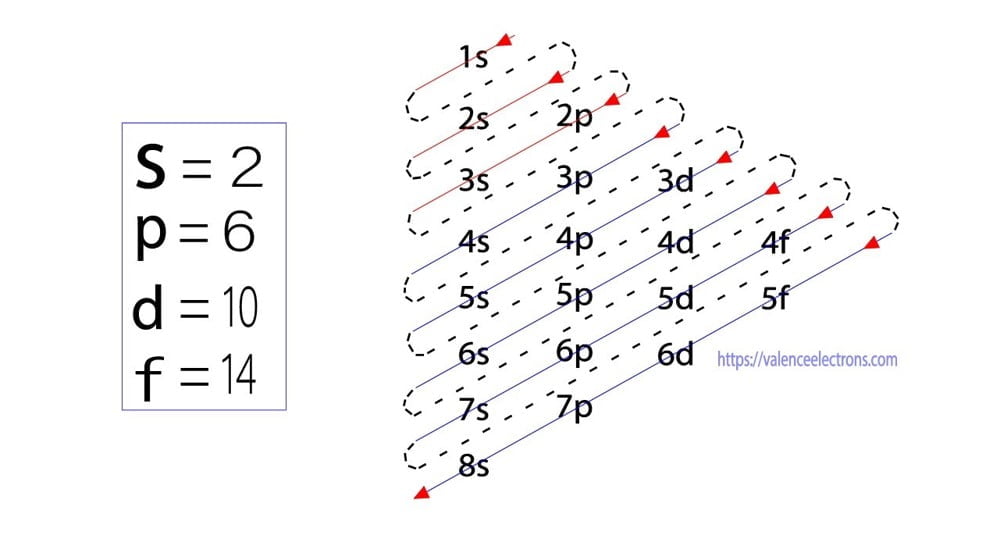
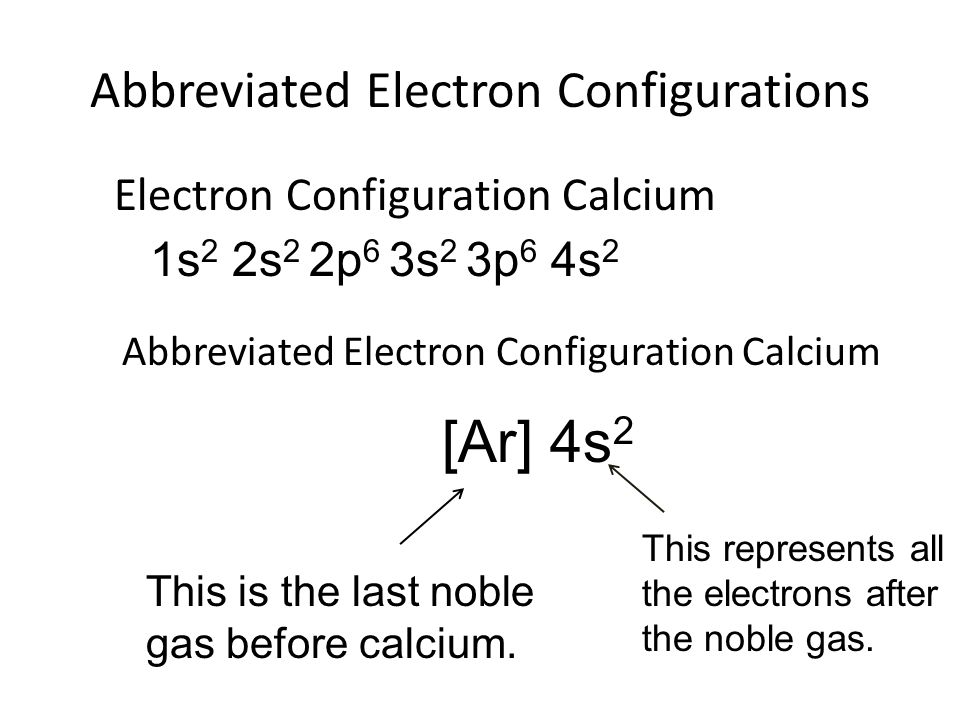
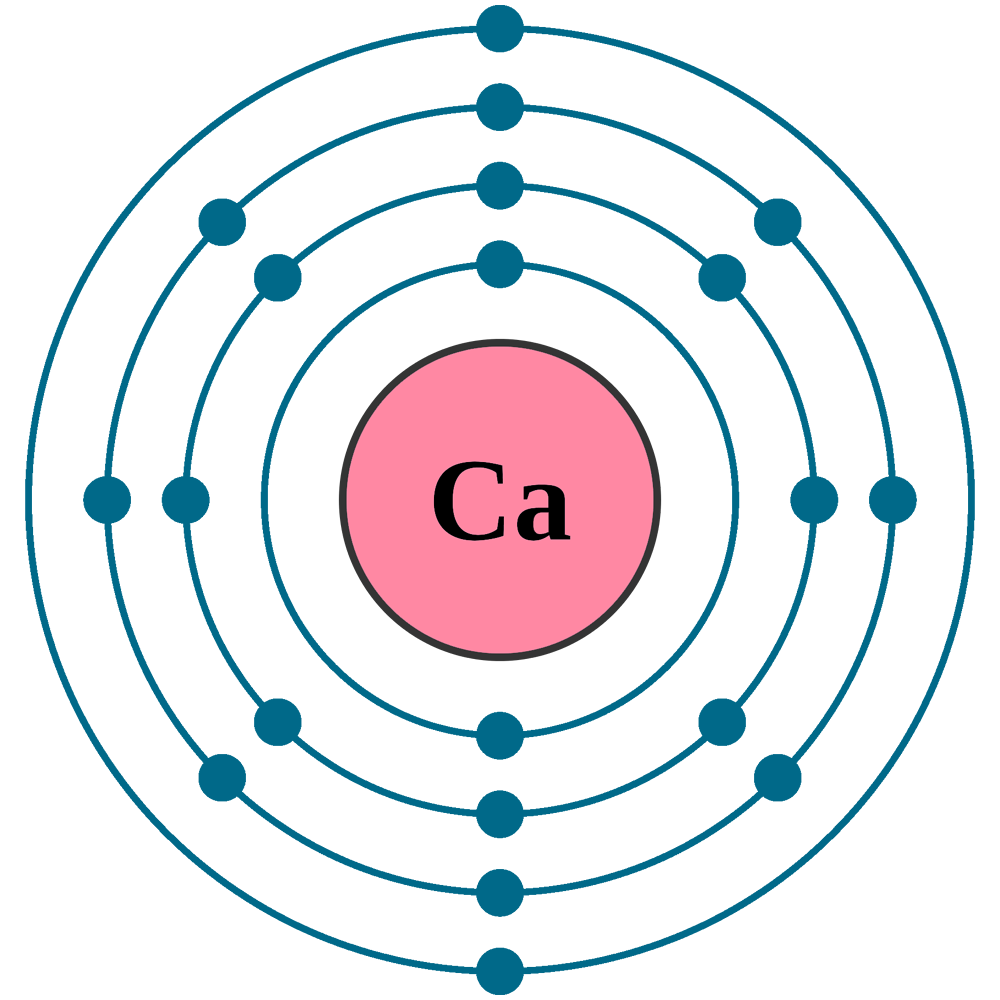
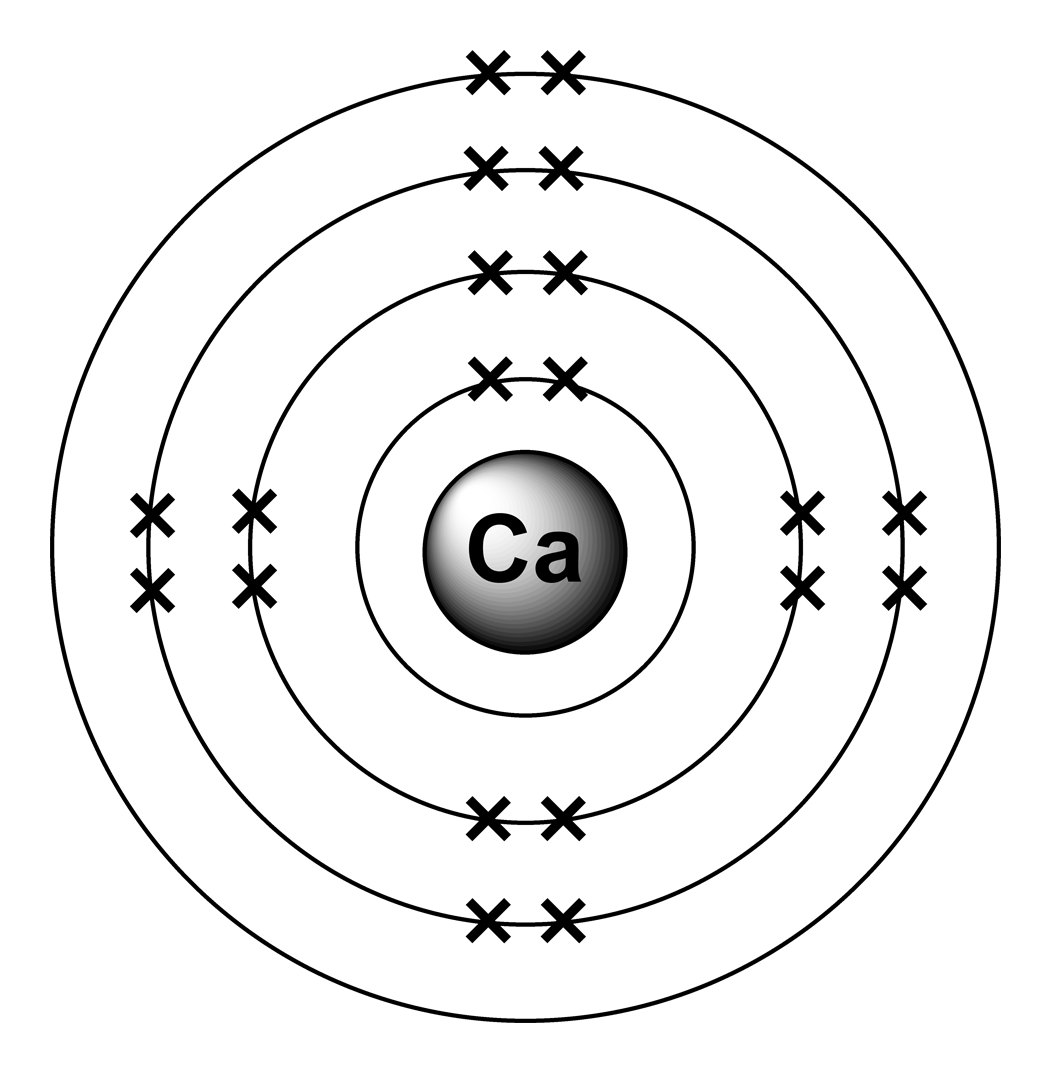
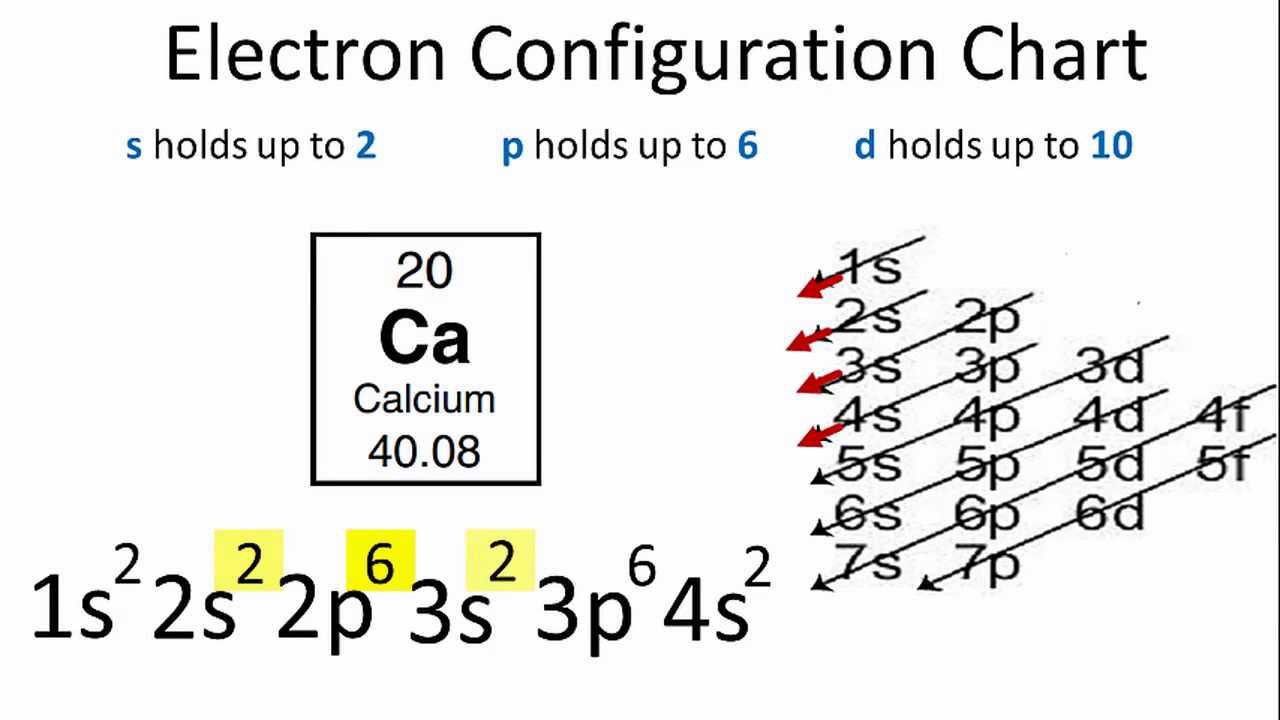
What Is The Electron Configuration For Ca - Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. The shorthand electron configuration (or noble gas. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). Since we need to take away two electrons, we first remove electrons from the. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Electron configuration chart of all elements is mentioned in the table below. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as.
The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Since we need to take away two electrons, we first remove electrons from the. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2:
The shorthand electron configuration (or noble gas. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Electron configuration chart of all elements is mentioned in the table below. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: Since we need to take away two electrons, we first remove electrons from the.
How to Write the Electron Configuration for Calcium (Ca)
Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. The shorthand electron configuration (or noble gas. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons)..
Calcium Electron Configuration (Ca) with Orbital Diagram
The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Calcium is a chemical element of the.
Calcium Ca (Element 20) of Periodic Table Elements FlashCards
The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. In order to write.
ca orbital diagram TravisMatteo
The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Electron configuration chart of all elements is mentioned in the table below. Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p.
Calcium electron configuration Stock Image C029/5027 Science
Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Electron configuration chart of all elements is mentioned in the table below. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: Since we need to take away two electrons, we first remove electrons from the. Calcium.
Calcium electronic configuration How to Write Calcium electronic
Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\). Since we need to take away two electrons, we first remove electrons from the. The shorthand electron configuration (or noble gas. Hence,.
Electron Configuration for Calcium (Ca, Ca2+ ion)
The shorthand electron configuration (or noble gas. Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. Since we need to take away two.
Electron arrangements
Since we need to take away two electrons, we first remove electrons from the. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. In order to write the calcium electron configuration we first need to know the number of electrons for.
SOLVED Write the condensed (noblegas) electron configuration of Ca.
Since we need to take away two electrons, we first remove electrons from the. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: The shorthand electron.
Calcium Electron Configuration YouTube
The shorthand electron configuration (or noble gas. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons)..
The Shorthand Electron Configuration (Or Noble Gas.
Hence, the electron configuration for ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 [ar] 4s 2: Since we need to take away two electrons, we first remove electrons from the. Calcium is a chemical element of the periodic table with chemical symbol ca and atomic number 20 with an atomic weight of 40.0784 u and is classed as.
The Electron Configuration Of Sodium Is \(1S^2 2S^2 2P^6 3S^1\).
In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Electron configuration chart of all elements is mentioned in the table below.