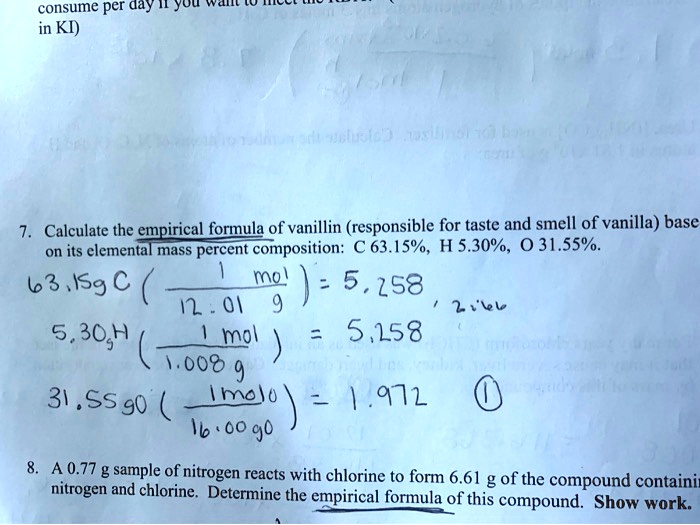What Is The Empirical Formula Of Vanillin
What Is The Empirical Formula Of Vanillin - When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? The empirical formula of vanillin: Calculation of the number of moles of c, h, and o in vanillin: Vanillin, a flavoring agent, is made up of carbon, hydrogen,. What is the empirical formula? Vanillin, the dominant flavoring in vanilla, contains c, h, and o.
Vanillin, the dominant flavoring in vanilla, contains c, h, and o. What is the empirical formula? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. The empirical formula of vanillin: What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? Vanillin, a flavoring agent, is made up of carbon, hydrogen,. When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. Calculation of the number of moles of c, h, and o in vanillin:
Calculation of the number of moles of c, h, and o in vanillin: Vanillin, the dominant flavoring in vanilla, contains c, h, and o. When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. What is the empirical formula? Vanillin, a flavoring agent, is made up of carbon, hydrogen,. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. The empirical formula of vanillin:
SOLVEDThe flavoring agent vanillin contains carbon, hydrogen, and
Vanillin, a flavoring agent, is made up of carbon, hydrogen,. Vanillin, the dominant flavoring in vanilla, contains c, h, and o. What is the empirical formula? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. The empirical formula of vanillin:
SOLVED 7.0 Calculate the empirical formula of vanillin (responsible
Calculation of the number of moles of c, h, and o in vanillin: Vanillin, the dominant flavoring in vanilla, contains c, h, and o. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. Vanillin, a flavoring agent, is made up of carbon,.
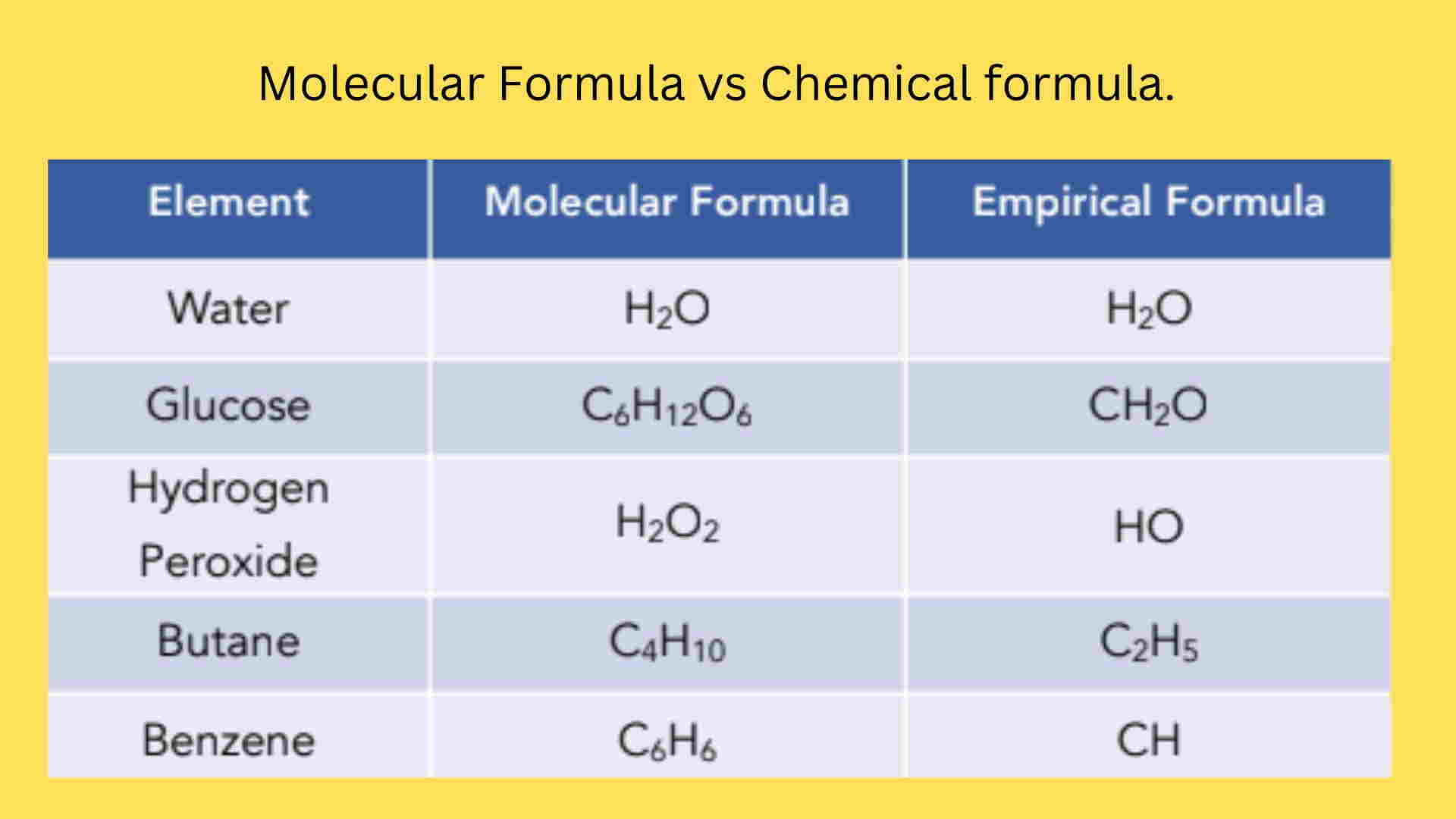
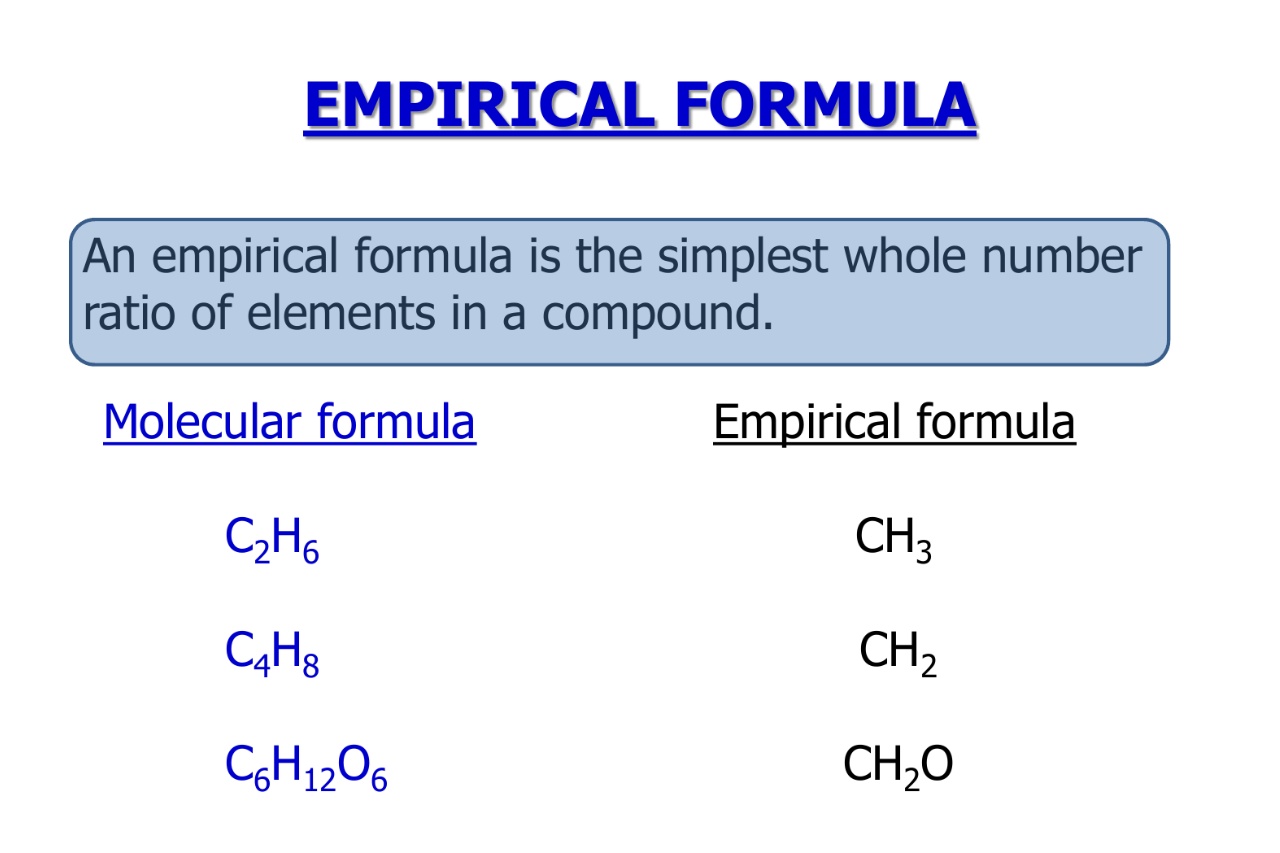
Empirical Formula, Molecular Formula Examples, Calculations
Vanillin, a flavoring agent, is made up of carbon, hydrogen,. When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. Calculation of the number of moles of c, h, and o in vanillin: Vanillin, the dominant flavoring in vanilla, contains c, h, and o. What are the empirical formula and empirical formula mass for.
15 Astounding Facts About Empirical Formula
What is the empirical formula? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. The empirical formula of vanillin: What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? Calculation of the number of moles of c, h, and o in vanillin:
[Solved] Empirical Formula Vanillin is the compound containing C, H
When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. What is the empirical formula? Vanillin, a flavoring agent, is made up of carbon, hydrogen,. Vanillin, the dominant flavoring in vanilla, contains c, h, and o.
What is the empirical formula of Vanillin? HIX Tutor
What is the empirical formula? Calculation of the number of moles of c, h, and o in vanillin: A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. Vanillin, a flavoring agent, is made up of carbon, hydrogen,. The empirical formula of vanillin:
Solved Determine the empirical formula of vanillin, a
Vanillin, a flavoring agent, is made up of carbon, hydrogen,. What is the empirical formula? Vanillin, the dominant flavoring in vanilla, contains c, h, and o. A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g.
[Solved] Empirical Formula Vanillin is the compound containing C, H
The empirical formula of vanillin: What is the empirical formula? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. Vanillin, a flavoring agent, is made up of carbon, hydrogen,. Calculation of the number of moles of c, h, and o in vanillin:
SOLVED Calculate the empirical formula for each natural flavor based
The empirical formula of vanillin: Vanillin, a flavoring agent, is made up of carbon, hydrogen,. Vanillin, the dominant flavoring in vanilla, contains c, h, and o. What is the empirical formula? Calculation of the number of moles of c, h, and o in vanillin:
[Solved] Empirical Formula Vanillin is the compound containing C, H
When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? Vanillin, a flavoring agent, is made up of carbon, hydrogen,. A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. What is the empirical formula?
Vanillin, A Flavoring Agent, Is Made Up Of Carbon, Hydrogen,.
Calculation of the number of moles of c, h, and o in vanillin: A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. The empirical formula of vanillin: What are the empirical formula and empirical formula mass for #c_8h_24 0_8#?
Vanillin, The Dominant Flavoring In Vanilla, Contains C, H, And O.
When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. What is the empirical formula?