What Is The Hybridization Of The Central Atom In Nh3
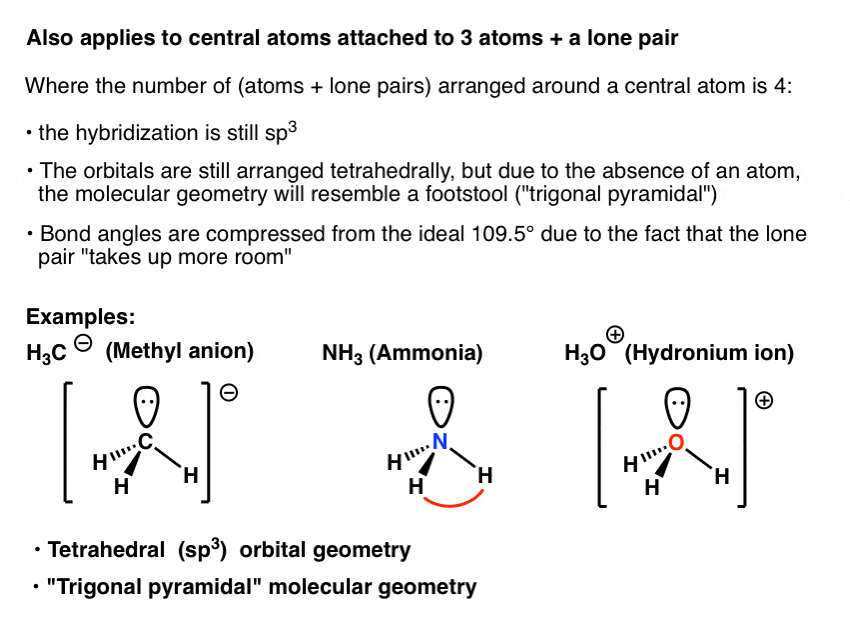
What Is The Hybridization Of The Central Atom In Nh3 - We'll look at how to figure out if nh 3 is. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.
The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. We'll look at how to figure out if nh 3 is.
Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. We'll look at how to figure out if nh 3 is. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³.
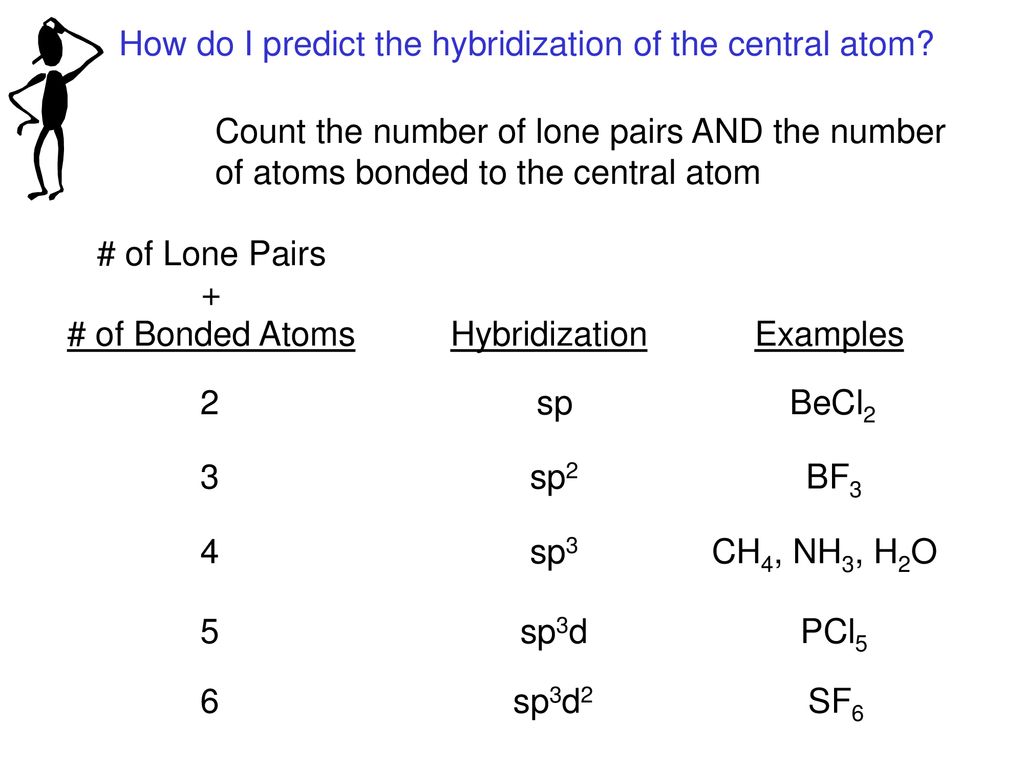
The correct order of hybridization of the central atom in the following
The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. We'll look at how to figure out if nh 3 is.
Experiments show O2 is ppt download
The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. We'll look at how to figure out if nh 3 is.
Hybridization and Hybrid Orbitals ChemTalk
We'll look at how to figure out if nh 3 is. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.
SOLVED Number of bonding electron pairs (bp) Molecular Geometry
Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. We'll look at how to figure out if nh 3 is.
What Are Hybrid Orbitals and Hybridization? Master Organic Chemistry
We'll look at how to figure out if nh 3 is. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³.
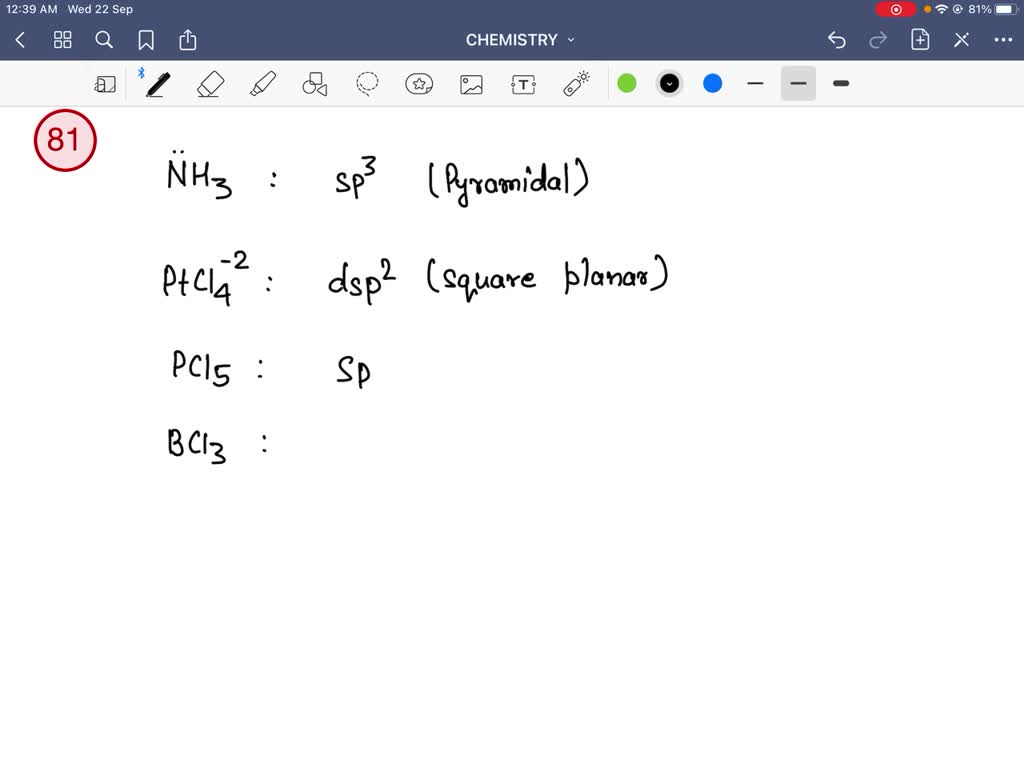
14. (a) What is the hybridization of central atom in following? NH3 ,C2 H..
Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. We'll look at how to figure out if nh 3 is. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³.
The correct order of hybridization of the central atom in the following
Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. We'll look at how to figure out if nh 3 is. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³.
What is the hybridization of the central atom in NH3? Hybridization
Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. We'll look at how to figure out if nh 3 is.
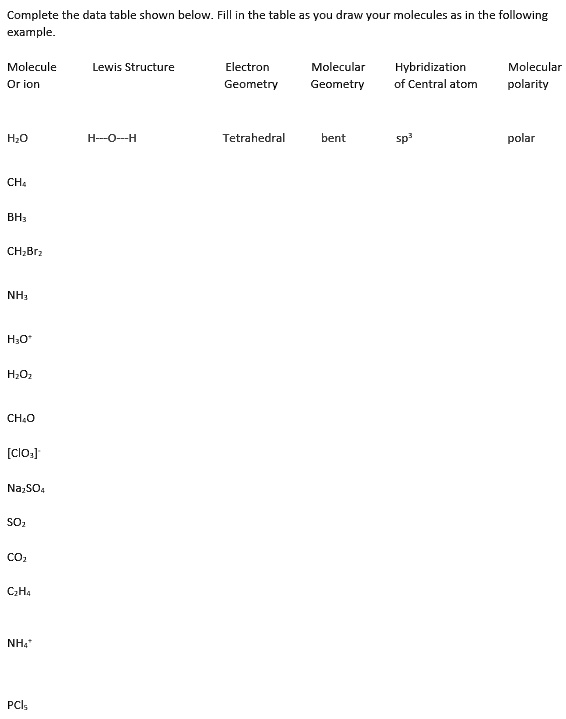
SOLVED Complete the data table shown below. Fill the table example
Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. We'll look at how to figure out if nh 3 is. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³.
[ANSWERED] A. What is the hybridization of the central atom in IF5
Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. We'll look at how to figure out if nh 3 is. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³.
We'll Look At How To Figure Out If Nh 3 Is.
The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.





![[ANSWERED] A. What is the hybridization of the central atom in IF5](https://media.kunduz.com/media/sug-question/raw/52262186-1659250506.6599846.jpeg?h=512)