What Two Substances Form From An Acid Base Neutralization
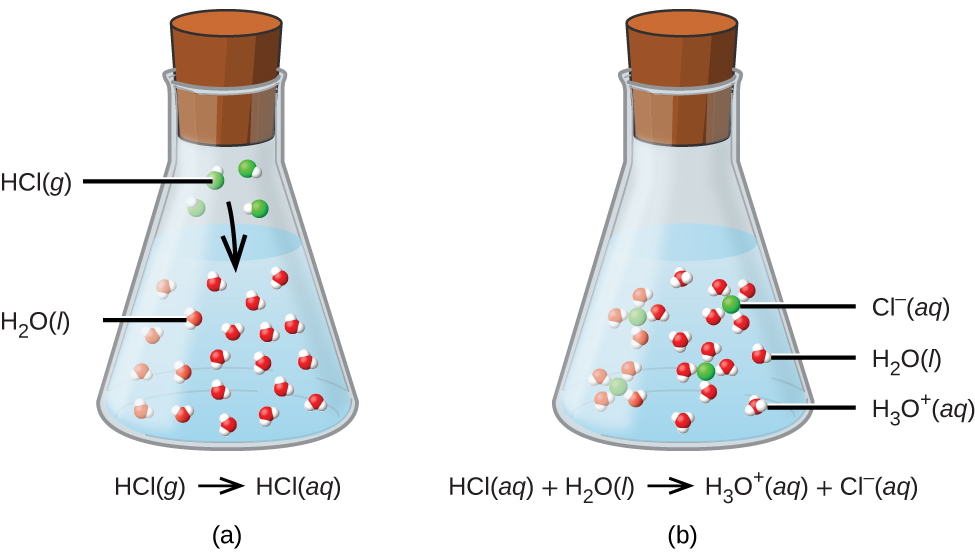
What Two Substances Form From An Acid Base Neutralization - In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called a salt. Acids and bases have another property:
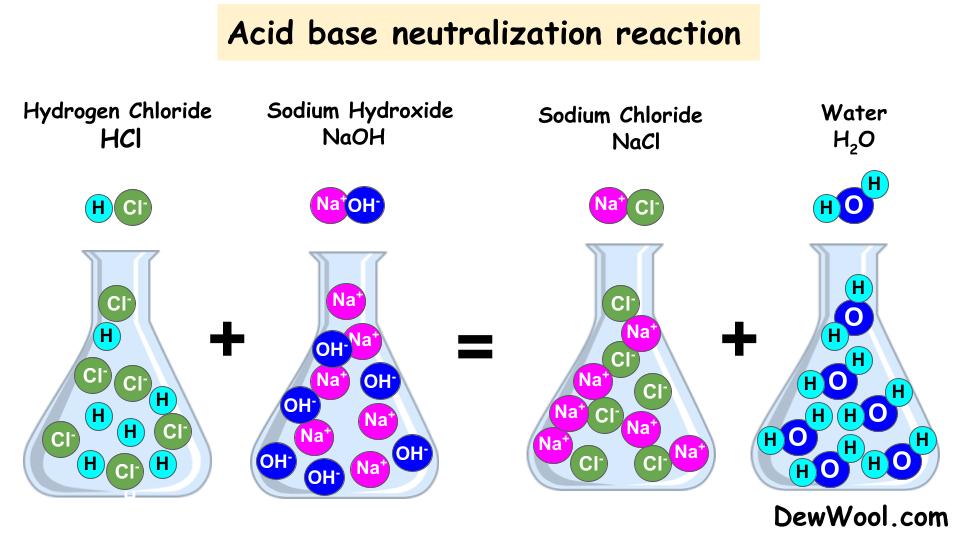
In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. Acids and bases have another property: They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity.
In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called a salt. Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity.
Concepts of Acid Base Neutralization PDF Acid Ph
In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called a salt. Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an.
Neutralization Reaction Diagram
Acids and bases have another property: They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and.
Neutralization Reaction Definition, Equation and Examples Teachoo
In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called a salt. Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an.
Acid/Base Neutralization Reactions & Net Ionic Equations YouTube
Acids and bases have another property: In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an.
What is Neutralisation in Chemistry? The Chemistry Blog
In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. They react with each other to make water and an ionic compound called.
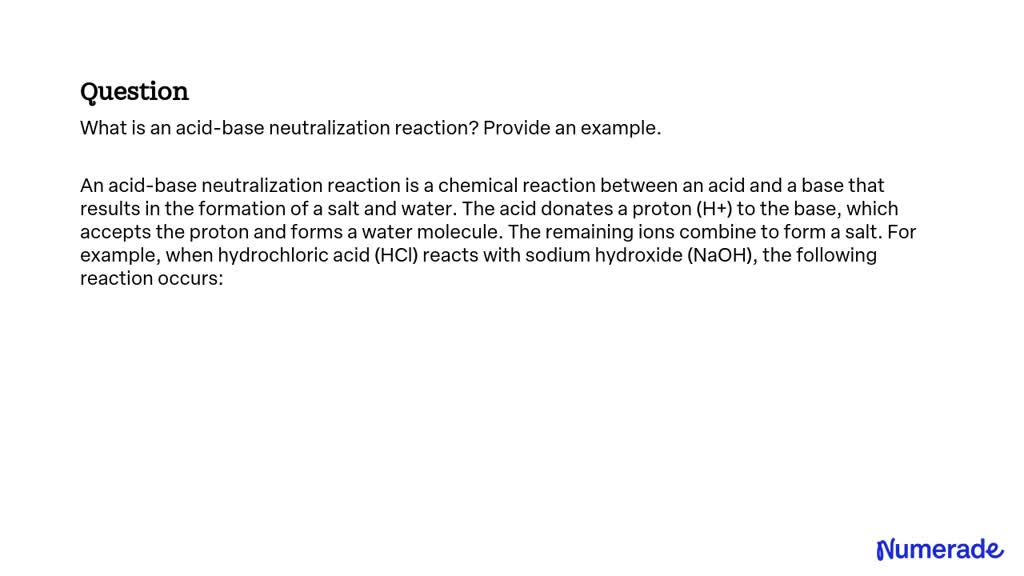
SOLVED What is an acidbase neutralization reaction? Provide an example.
They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. Acids and bases have.
Type Of Acid Base Neutralization Reactions
Acids and bases have another property: In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an.
Acid Base Neutralization Reactions Chemistry YouTube
In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. They react with each other to make water and an ionic compound called a salt. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. Acids and bases have.
SOLVED Which substances are always produced in an acidbase
They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. Acids and bases have.
Acidbase neutralization reaction, illustration Stock Image F027
They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. Acids and bases have.
Acids And Bases Have Another Property:
They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base.