Worksheet Ph And Poh Calculations
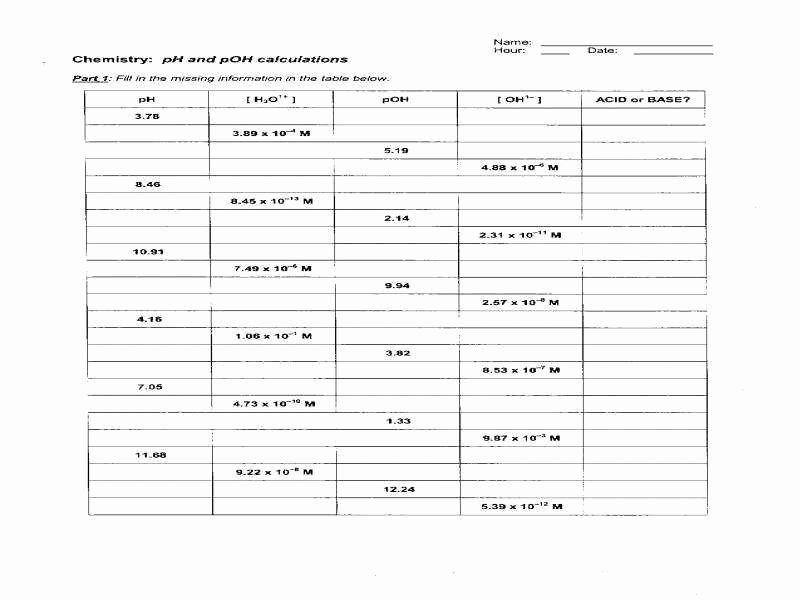
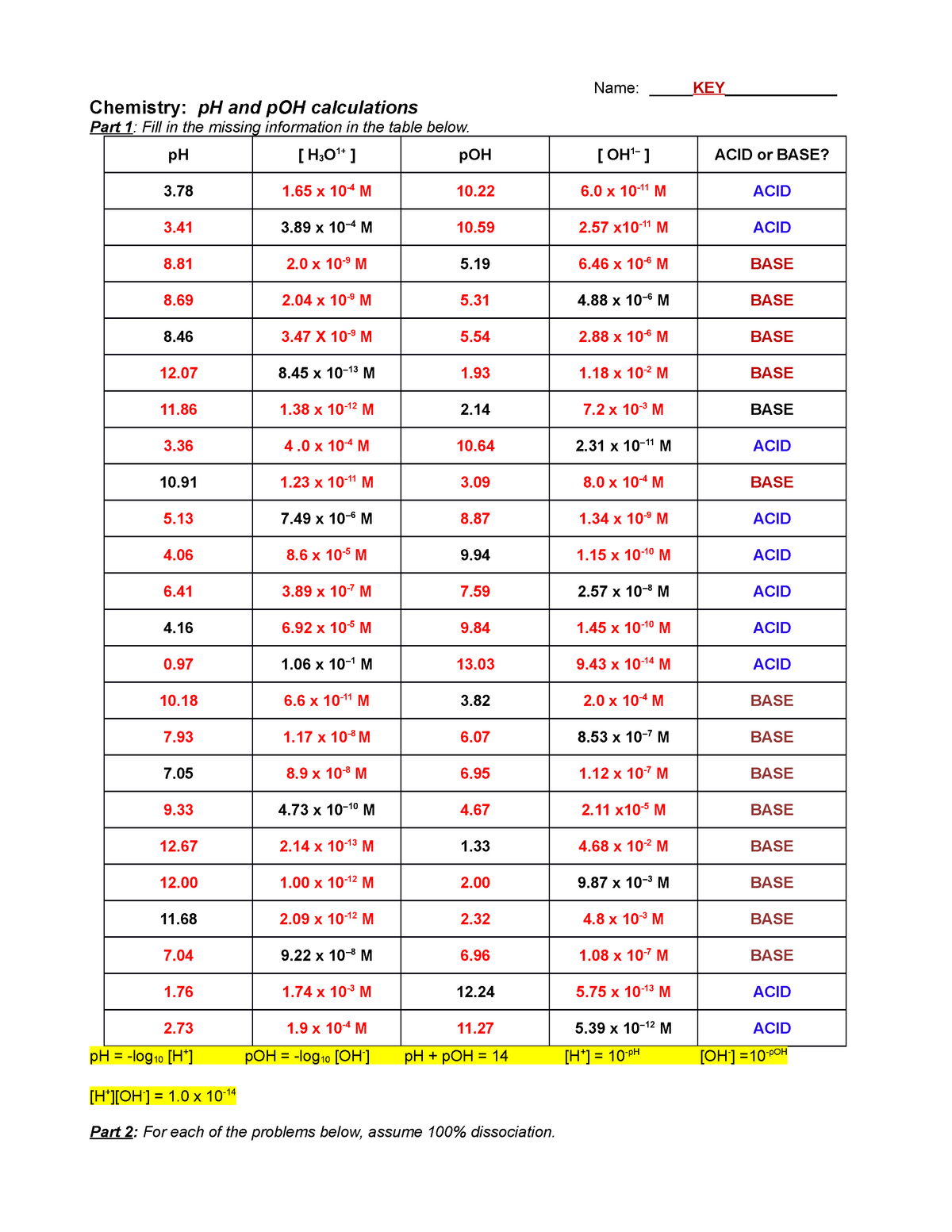
Worksheet Ph And Poh Calculations - Is it an acidic or basic solution? It is an acidic solution because ph = 3.89 < 7 2. Solve the following ph calculations. Fill in the missing information in the table below. What is the concentration of [h+] in a solution whose ph = 4.3? Write the formula, plug numbers into formula, & give answer with correct units and significant. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. If the hydroxide ion concentration. For each of the problems below, assume 100% dissociation.
Write the formula, plug numbers into formula, & give answer with correct units and significant. What is the concentration of [h+] in a solution whose ph = 4.3? Solve the following ph calculations. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Fill in the missing information in the table below. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Ph and poh practice d. It is an acidic solution because ph = 3.89 < 7 2. For each of the problems below, assume 100% dissociation. Is it an acidic or basic solution?
Is it an acidic or basic solution? What is the concentration of [h+] in a solution whose ph = 4.3? Fill in the missing information in the table below. If the hydroxide ion concentration. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. For each of the problems below, assume 100% dissociation. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Write the formula, plug numbers into formula, & give answer with correct units and significant. It is an acidic solution because ph = 3.89 < 7 2. Ph and poh practice d.
42 ph and poh worksheet Worksheet Master
Write the formula, plug numbers into formula, & give answer with correct units and significant. It is an acidic solution because ph = 3.89 < 7 2. Fill in the missing information in the table below. If the hydroxide ion concentration. Is it an acidic or basic solution?
Ph And Poh Worksheet E Street Light
Ph and poh practice d. Write the formula, plug numbers into formula, & give answer with correct units and significant. For each of the problems below, assume 100% dissociation. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Is it an acidic or basic solution?
Ph And Poh Worksheet E Street Light
Write the formula, plug numbers into formula, & give answer with correct units and significant. It is an acidic solution because ph = 3.89 < 7 2. Is it an acidic or basic solution? Solve the following ph calculations. If the hydroxide ion concentration.
50 Ph And Poh Worksheet Answers
Is it an acidic or basic solution? What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Write the formula, plug numbers into formula, & give answer with correct units and significant. If the hydroxide ion concentration. For each of the problems below, assume 100% dissociation.
Ph And Poh Worksheet Answers
Solve the following ph calculations. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. What is the concentration of [h+] in a solution whose ph = 4.3? For each of the problems below, assume 100% dissociation. Is it an acidic or basic solution?
Ph And Poh Calculations Worksheet Answer Key CALCULATOR CGW
Write the formula, plug numbers into formula, & give answer with correct units and significant. Fill in the missing information in the table below. Solve the following ph calculations. What is the concentration of [h+] in a solution whose ph = 4.3? If the hydroxide ion concentration.
50 Ph And Poh Worksheet Answers
If the hydroxide ion concentration. Write the formula, plug numbers into formula, & give answer with correct units and significant. For each of the problems below, assume 100% dissociation. Ph and poh practice d. What is the ph of a solution that has a hydronium concentration of 3.4 x 10.
The pH Scale Calculations with pH and pOH Notes and Worksheet Set
It is an acidic solution because ph = 3.89 < 7 2. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. If the hydroxide ion concentration. For each of the problems below, assume 100% dissociation. Is it an acidic or basic solution?
10++ Ph And Poh Calculations Worksheet Worksheets Decoomo
Fill in the missing information in the table below. Solve the following ph calculations. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. If the hydroxide ion concentration. What is the concentration of [h+] in a solution whose ph = 4.3?
34+ ph and poh calculations worksheet PeterGrantas
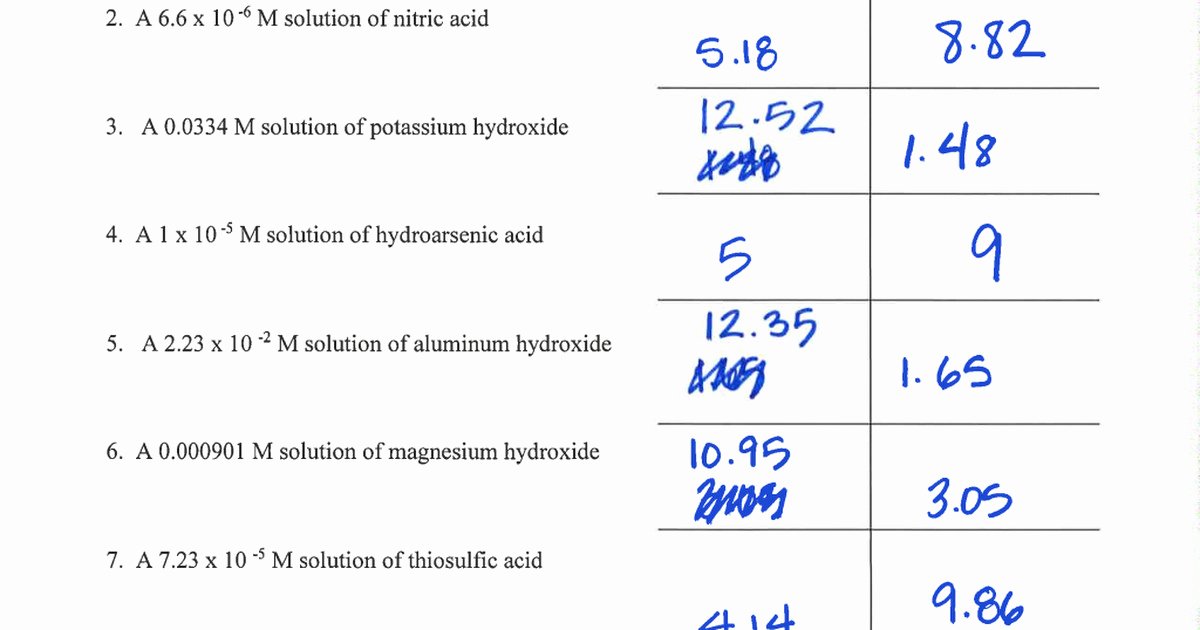
Write the formula, plug numbers into formula, & give answer with correct units and significant. For each of the problems below, assume 100% dissociation. If the hydroxide ion concentration. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Solve the following ph calculations.
Is It An Acidic Or Basic Solution?
What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Fill in the missing information in the table below. If the hydroxide ion concentration. Ph and poh practice d.
Write The Formula, Plug Numbers Into Formula, & Give Answer With Correct Units And Significant.
What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Solve the following ph calculations. What is the concentration of [h+] in a solution whose ph = 4.3? It is an acidic solution because ph = 3.89 < 7 2.



:max_bytes(150000):strip_icc()/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)